Effects of positive allosteric modulators on single-cell oscillatory Ca2+ signaling initiated by the type 5 metabotropic glutamate receptor
- PMID: 19737913
- PMCID: PMC2784724
- DOI: 10.1124/mol.109.059170
Effects of positive allosteric modulators on single-cell oscillatory Ca2+ signaling initiated by the type 5 metabotropic glutamate receptor
Abstract
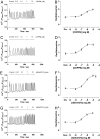
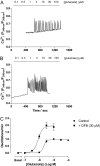
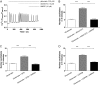
Agonist stimulation of the type 5 metabotropic glutamate (mGlu5) receptor initiates robust oscillatory changes in cytosolic Ca2+ concentration ([Ca2+]i) in single cells by rapid, repeated cycles of phosphorylation/dephosphorylation of the mGlu5 receptor, involving protein kinase C and as-yet-unspecified protein phosphatase activities. An emergent property of this type of Ca2+ oscillation-generating mechanism (termed "dynamic uncoupling") is that once a threshold concentration has been reached to initiate the Ca2+ oscillation, its frequency is largely insensitive to further increases in orthosteric agonist concentration. Here, we report the effects of positive allosteric modulators (PAMs) on the patterns of single-cell Ca2+ signaling in recombinant and native mGlu5 receptor-expressing systems. In a Chinese hamster ovary cell-line (CHO-lac-mGlu5a), none of the mGlu5 receptor PAMs studied [3,3'-difluorobenzaldazine (DFB), N-{4-chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl) methyl]phenyl}-2-hydroxy-benzamide (CPPHA), 3-cyano-N-(1, 3-diphenyl-1H-prazol-5-yl)benzamide (CDPPB), S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1,2,4]oxadiazol-5-yl]-piperidinl-1-yl}-methanone (ADX47273)], stimulated a Ca2+ response when applied alone, but each PAM concentration-dependently increased the frequency, without affecting the amplitude, of Ca2+ oscillations induced by glutamate or quisqualate. Therefore, PAMs can cause graded increases (and negative allosteric modulator-graded decreases) in the Ca2+ oscillation frequency stimulated by orthosteric agonist. Initial data in rat cerebrocortical astrocytes demonstrated that similar effects of PAMs could be observed in a native cell background, although at high orthosteric agonist concentrations, PAM addition could much more often be seen to drive rapid Ca2+ oscillations into peak-plateau responses. These data demonstrate that allosteric modulators can "tune" the Ca2+ oscillation frequency initiated by mGlu5 receptor activation, and this might allow pharmacological modification of the downstream processes (e.g., transcriptional regulation) that is unachievable through orthosteric ligand interactions.
Figures




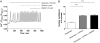



References
-
- Atkinson PJ, Young KW, Ennion SJ, Kew JN, Nahorski SR, Challiss RA. (2006) Altered expression of Gq/11α protein shapes mGlu1 and mGlu5 receptor-mediated single cell inositol 1,4,5-trisphosphate and Ca2+ signaling. Mol Pharmacol 69:174–184 - PubMed
-
- Berridge MJ, Lipp P, Bootman MD. (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1:11–21 - PubMed
-
- Chen Y, Nong Y, Goudet C, Hemstapat K, de Paulis T, Pin JP, Conn PJ. (2007) Interaction of novel positive allosteric modulators of metabotropic glutamate receptor 5 with the negative allosteric antagonist site is required for potentiation of receptor responses. Mol Pharmacol 71:1389–1398 - PubMed
-
- Chen Y, Goudet C, Pin JP, Conn PJ. (2008) N-{4-Chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl}-2-hydroxybenzamide (CPPHA) acts through a novel site as a positive allosteric modulator of group 1 metabotropic glutamate receptors. Mol Pharmacol 73:909–918 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

