Interaction of organic cations with organic anion transporters
- PMID: 19737926
- PMCID: PMC2781538
- DOI: 10.1074/jbc.M109.024489
Interaction of organic cations with organic anion transporters
Abstract
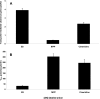
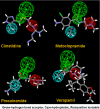
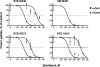
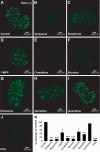
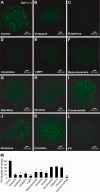
Studies of the organic anion transporters (Oats) have focused mainly on their interactions with organic anionic substrates. However, as suggested when Oat1 was originally identified as NKT (Lopez-Nieto, C. E., You, G., Bush, K. T., Barros, E. J., Beier, D. R., and Nigam, S. K. (1997) J. Biol. Chem. 272, 6471-6478), since the Oats share close homology with organic cation transporters (Octs), it is possible that Oats interact with cations as well. We now show that mouse Oat1 (mOat1) and mOat3 and, to a lesser degree, mOat6 bind a number of "prototypical" Oct substrates, including 1-methyl-4-phenylpyridinium. In addition to oocyte expression assays, we have tested binding of organic cations to Oat1 and Oat3 in ex vivo assays by analyzing interactions in kidney organ cultures deficient in Oat1 and Oat3. We also demonstrate that mOat3 transports organic cations such as 1-methyl-4-phenylpyridinium and cimetidine. A pharmacophore based on the binding affinities of the tested organic cations for Oat3 was generated. Using this pharmacophore, we screened a chemical library and were able to identify novel cationic compounds that bound to Oat1 and Oat3. These compounds bound Oat3 with an affinity higher than the highest affinity compounds in the original set of prototypical Oct substrates. Thus, whereas Oat1, Oat3, and Oat6 appear to function largely in organic anion transport, they also bind and transport some organic cations. These findings could be of clinical significance, since drugs and metabolites that under normal physiological conditions do not bind to the Oats may undergo changes in charge and become Oat substrates during pathologic conditions wherein significant variations in body fluid pH occur.
Figures

References
-
- Lopez-Nieto C. E., You G., Bush K. T., Barros E. J., Beier D. R., Nigam S. K. (1997) J. Biol. Chem. 272, 6471–6478 - PubMed
-
- Sweet D. H., Chan L. M., Walden R., Yang X. P., Miller D. S., Pritchard J. B. (2003) Am. J. Physiol. Renal Physiol. 284, F763–F769 - PubMed
-
- Sweet D. H., Wolff N. A., Pritchard J. B. (1997) J. Biol. Chem. 272, 30088–30095 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

