C-Raf is associated with disease progression and cell proliferation in a subset of melanomas
- PMID: 19737955
- PMCID: PMC2763114
- DOI: 10.1158/1078-0432.CCR-09-0198
C-Raf is associated with disease progression and cell proliferation in a subset of melanomas
Abstract
Purpose: Raf-kinases include three major isoforms. Although the role of B-Raf in melanoma is well established, little is known about C-Raf. We studied effects of C-Raf knockdown in vitro and assessed expression of C-Raf in a large cohort of melanomas and nevi.
Experimental design: Using specific siRNAs, we knocked down C-Raf expression, and determined the effect on viability, MAP extracellular signal-regulated kinase (ERK)/ERK kinase signaling, and apoptosis in seven melanoma cell lines. We determined the IC(50) of the C-Raf inhibitors sorafenib and GW5074, and studied the effects of GW5074 on cell signaling. Using an automated method to measure in situ protein expression, we quantified C-Raf expression in 263 nevi and 523 melanomas.
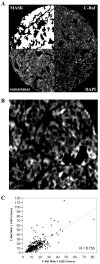
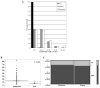
Results: C-Raf was knocked down in three cell lines with detectable phospho-C-Raf, resulting in decreased viability in two of the three (YULAC and YUROB). This resulted in decreased Bcl-2 expression and phospho-Bad cleavage, without affecting phospho-MEK and phospho-ERK. Sensitivity to sorafenib and GW5074 varied. GW5074 inhibited mitogen-activated protein kinase signaling without Bcl-2 and phospho-Bad down-regulation. C-Raf was highly expressed in melanomas compared with nevi (P < 0.0001), and no nevi had high C-Raf expression. C-Raf expression was higher in metastatic than primary specimens (P = 0.0225).
Conclusions: C-Raf siRNA knock-down results in decreased viability of YULAC (B-Raf(V600K)) and YUROB (B-Raf(WT)) melanoma cells, likely mediated by Bcl-2 inhibition rather than mitogen-activated protein kinase inhibition. Cotargeting C-Raf and parallel pathways might be an effective therapeutic approach for melanoma. C-Raf expression is up-regulated in a subset of melanomas but not in nevi, suggesting that it might be a valuable diagnostic marker and therapeutic target.
Figures
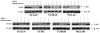


References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Nashan D, Muller ML, Grabbe S, Wustlich S, Enk A. Systemic therapy of disseminated malignant melanoma: an evidence-based overview of the state-of-the-art in daily routine. J Eur Acad Dermatol Venereol. 2007;21:1305–18. - PubMed
-
- Smalley KS. A pivotal role for ERK in the oncogenic behaviour of malignant melanoma? Int J Cancer. 2003;104:527–32. - PubMed
-
- Dhawan P, Singh AB, Ellis DL, Richmond A. Constitutive activation of Akt/protein kinase B in melanoma leads to up-regulation of nuclear factor-kappaB and tumor progression. Cancer Res. 2002;62:7335–42. - PubMed
-
- Kolch W, Kotwaliwale A, Vass K, Janosch P. The role of Raf kinases in malignant transformation. Expert Rev Mol Med. 2002;4:1–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

