Tuberous sclerosis complex suppression in cerebellar development and medulloblastoma: separate regulation of mammalian target of rapamycin activity and p27 Kip1 localization
- PMID: 19738049
- PMCID: PMC2745891
- DOI: 10.1158/0008-5472.CAN-09-1299
Tuberous sclerosis complex suppression in cerebellar development and medulloblastoma: separate regulation of mammalian target of rapamycin activity and p27 Kip1 localization
Abstract
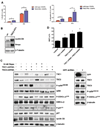
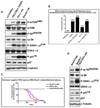
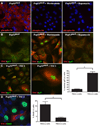
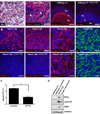
During development, proliferation of cerebellar granule neuron precursors (CGNP), candidate cells-of-origin for the pediatric brain tumor medulloblastoma, requires signaling by Sonic hedgehog (Shh) and insulin-like growth factor (IGF), the pathways of which are also implicated in medulloblastoma. One of the consequences of IGF signaling is inactivation of the mammalian target of rapamycin (mTOR)-suppressing tuberous sclerosis complex (TSC), comprised of TSC1 and TSC2, leading to increased mRNA translation. We show that mice, in which TSC function is impaired, display increased mTOR pathway activation, enhanced CGNP proliferation, glycogen synthase kinase-3 alpha/beta (GSK-3 alpha/beta) inactivation, and cytoplasmic localization of the cyclin-dependent kinase inhibitor p27(Kip1), which has been proposed to cause its inactivation or gain of oncogenic functions. We observed the same characteristics in wild-type primary cultures of CGNPs in which TSC1 and/or TSC2 were knocked down, and in mouse medulloblastomas induced by ectopic Shh pathway activation. Moreover, Shh-induced mouse medulloblastomas manifested Akt-mediated TSC2 inactivation, and the mutant TSC2 allele synergized with aberrant Shh signaling to increase medulloblastoma incidence in mice. Driving exogenous TSC2 expression in Shh-induced medulloblastoma cells corrected p27(Kip1) localization and reduced proliferation. GSK-3 alpha/beta inactivation in the tumors in vivo and in primary CGNP cultures was mTOR-dependent, whereas p27(Kip1) cytoplasmic localization was regulated upstream of mTOR by TSC2. These results indicate that a balance between Shh mitogenic signaling and TSC function regulating new protein synthesis and cyclin-dependent kinase inhibition is essential for the normal development and prevention of tumor formation or expansion.
Conflict of interest statement
Figures

References
-
- Rubin JB, Rowitch DH. Medulloblastoma: a problem of developmental biology. Cancer Cell. 2002;2:7–8. - PubMed
-
- Knoepfler PS, Kenney AM. Neural precursor cycling at sonic speed: N-Myc pedals, GSK-3 brakes. Cell Cycle. 2006;5:47–52. - PubMed
-
- Wetmore C. Sonic hedgehog in normal and neoplastic proliferation: insight gained from human tumors and animal models. Curr Opin Genet Dev. 2003;13:34–42. - PubMed
-
- Pietsch T, Waha A, Koch A, et al. Medulloblastomas of the desmoplastic variant carry mutations of the human homologue of Drosophila patched. Cancer Res. 1997;57:2085–2088. - PubMed
-
- Raffel C, Jenkins RB, Frederick L, et al. Sporadic medulloblastomas contain PTCH mutations. Cancer Res. 1997;57:842–845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

