Melanoma proteoglycan modifies gene expression to stimulate tumor cell motility, growth, and epithelial-to-mesenchymal transition
- PMID: 19738072
- PMCID: PMC2762355
- DOI: 10.1158/0008-5472.CAN-08-4626
Melanoma proteoglycan modifies gene expression to stimulate tumor cell motility, growth, and epithelial-to-mesenchymal transition
Abstract
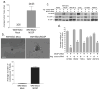
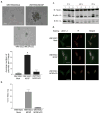
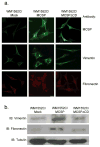
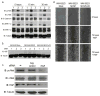
Melanoma chondroitin sulfate proteoglycan (MCSP) is a plasma membrane-associated proteoglycan that facilitates the growth, motility, and invasion of tumor cells. MCSP expression in melanoma cells enhances integrin function and constitutive activation of Erk1,2. The current studies were performed to determine the mechanism by which MCSP expression promotes tumor growth and motility. The results show that MCSP expression in radial growth phase, vertical growth phase, or metastatic cell lines causes sustained activation of Erk1,2, enhanced growth, and motility which all require the cytoplasmic domain of the MCSP core protein. MCSP expression in a radial growth phase cell line also promotes an epithelial-to-mesenchymal transition based on changes in cell morphology and the expression of several epithelial-to-mesenchymal transition markers. Finally, MCSP enhances the expression of c-Met and hepatocyte growth factor, and inhibiting c-Met expression or activation limits the increased growth and motility of multiple melanoma cell lines. The studies collectively show the importance of MCSP in promoting progression by an epigenetic mechanism and they indicate that MCSP could be targeted to delay or inhibit tumor progression in patients.
Figures

References
-
- Campoli MR, Chang CC, Kageshita T, Wang X, McCarthy JB, Ferrone S. Human high molecular weight-melanoma-associated antigen (HMW-MAA): a melanoma cell surface chondroitin sulfate proteoglycan (MSCP) with biological and clinical significance. Crit Rev Immunol. 2004;24:267–96. - PubMed
-
- Chekenya M, Rooprai HK, Davies D, Levine JM, Butt AM, Pilkington GJ. The NG2 chondroitin sulfate proteoglycan: role in malignant progression of human brain tumours. Int J Dev Neurosci. 1999;17:421–35. - PubMed
-
- Stallcup WB. The NG2 proteoglycan: past insights and future prospects. J Neurocytol. 2002;31:423–35. - PubMed
-
- Kageshita T, Nakamura T, Yamada M, Kuriya N, Arao T, Ferrone S. Differential expression of melanoma associated antigens in acral lentiginous melanoma and in nodular melanoma lesions. Cancer Res. 1991;51:1726–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

