Bacterial lipopolysaccharide enhances PDGF signaling and pulmonary fibrosis in rats exposed to carbon nanotubes
- PMID: 19738159
- PMCID: PMC2937228
- DOI: 10.1165/rcmb.2009-0113OC
Bacterial lipopolysaccharide enhances PDGF signaling and pulmonary fibrosis in rats exposed to carbon nanotubes
Abstract
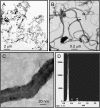
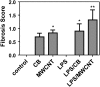
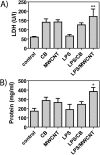
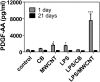
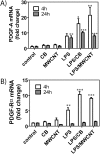
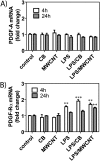
Engineered multi-walled carbon nanotubes (MWCNT) represent a possible health risk for pulmonary fibrosis due to their fiber-like shape and potential for persistence in the lung. We postulated that bacterial lipopolysaccharide (LPS), a ubiquitous agent in the environment that causes lung inflammation, would enhance fibrosis caused by MWCNT. Rats were exposed to LPS and then intratracheally instilled with MWCNT or carbon black (CB) nanoparticles 24 hours later. Pulmonary fibrosis was observed 21 days after MWCNT exposure, but not with CB. LPS alone caused no fibrosis but enhanced MWCNT-induced fibrosis. LPS plus CB did not significantly increase fibrosis. MWCNT increased platelet-derived growth factor-AA (PDGF-AA), a major mediator of fibrosis. PDGF-AA production in response to MWCNT, but not CB, was synergistically enhanced by LPS. Immunostaining showed PDGF-AA in bronchiolar epithelial cells and macrophages. Since macrophages engulfed MWCNT, were positive for PDGF-AA, and mediate fibroblast responses, experiments were performed with rat lung macrophages (NR8383 cells) and rat lung fibroblasts in vitro. LPS exposure increased PDGF-A mRNA levels in NR8383 cells and enhanced MWCNT-induced PDGF-A mRNA levels. Moreover, LPS increased MWCNT- or CB-induced PDGF receptor-alpha (PDGF-Ralpha) mRNA in fibroblasts. Our data suggest that LPS exacerbates MWCNT-induced lung fibrosis by amplifying production of PDGF-AA in macrophages and epithelial cells, and by increasing PDGF-Ralpha on pulmonary fibroblasts. Our findings also suggest that individuals with pre-existing pulmonary inflammation are at greater risk for the potential adverse effects of MWCNT.
Figures



References
-
- Baughman RH, Zakhidov AA, de Heer WA. Carbon nanotubes – the route toward applications. Science 2002;297:787–792. - PubMed
-
- Avouris P, Chen Z, Perebeinos V. Carbon-based electronics. Nat Nanotechnol 2007;2:605–615. - PubMed
-
- Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol 2005;9:674–679. - PubMed
-
- Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrest G, Alexander A. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci 2006;92:5–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

