Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer
- PMID: 19738166
- PMCID: PMC2758310
- DOI: 10.1093/jnci/djp280
Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer
Abstract
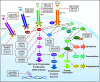
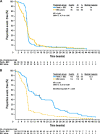
The monoclonal antibodies panitumumab and cetuximab that target the epidermal growth factor receptor (EGFR) have expanded the range of treatment options for metastatic colorectal cancer. Initial evaluation of these agents as monotherapy in patients with EGFR-expressing chemotherapy-refractory tumors yielded response rates of approximately 10%. The realization that detection of positive EGFR expression by immunostaining does not reliably predict clinical outcome of EGFR-targeted treatment has led to an intense search for alternative predictive biomarkers. Oncogenic activation of signaling pathways downstream of the EGFR, such as mutation of KRAS, BRAF, or PIK3CA oncogenes, or inactivation of the PTEN tumor suppressor gene is central to the progression of colorectal cancer. Tumor KRAS mutations, which may be present in 35%-45% of patients with colorectal cancer, have emerged as an important predictive marker of resistance to panitumumab or cetuximab treatment. In addition, among colorectal tumors carrying wild-type KRAS, mutation of BRAF or PIK3CA or loss of PTEN expression may be associated with resistance to EGFR-targeted monoclonal antibody treatment, although these additional biomarkers require further validation before incorporation into clinical practice. Additional knowledge of the molecular basis for sensitivity or resistance to EGFR-targeted monoclonal antibodies will allow the development of new treatment algorithms to identify patients who are most likely to respond to treatment and could also provide rationale for combining therapies to overcome primary resistance. The use of KRAS mutations as a selection biomarker for anti-EGFR monoclonal antibody (eg, panitumumab or cetuximab) treatment is the first major step toward individualized treatment for patients with metastatic colorectal cancer.
Figures


Comment in
-
Re: Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer.J Natl Cancer Inst. 2010 Apr 21;102(8):573; author reply 573-5. doi: 10.1093/jnci/djq064. Epub 2010 Mar 19. J Natl Cancer Inst. 2010. PMID: 20305131 No abstract available.
References
-
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–137. - PubMed
-
- Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res. 2006;12(18):5268–5272. - PubMed
-
- Harari PM. Epidermal growth factor receptor inhibition strategies in oncology. Endocr Relat Cancer. 2004;11(4):689–708. - PubMed
-
- Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37(suppl 4):S9–S15. - PubMed
-
- Mackenzie MJ, Hirte HW, Glenwood G, et al. A phase II trial of ZD1839 (Iressa) 750 mg per day, an oral epidermal growth factor receptor-tyrosine kinase inhibitor, in patients with metastatic colorectal cancer. Invest New Drugs. 2005;23(2):165–170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

