Proteomic analysis of integrin-associated complexes identifies RCC2 as a dual regulator of Rac1 and Arf6
- PMID: 19738201
- PMCID: PMC2857963
- DOI: 10.1126/scisignal.2000396
Proteomic analysis of integrin-associated complexes identifies RCC2 as a dual regulator of Rac1 and Arf6
Abstract
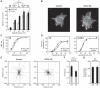
The binding of integrin adhesion receptors to their extracellular matrix ligands controls cell morphology, movement, survival, and differentiation in various developmental, homeostatic, and disease processes. Here, we report a methodology to isolate complexes associated with integrin adhesion receptors, which, like other receptor-associated signaling complexes, have been refractory to proteomic analysis. Quantitative, comparative analyses of the proteomes of two receptor-ligand pairs, alpha(4)beta(1)-vascular cell adhesion molecule-1 and alpha(5)beta(1)-fibronectin, defined both core and receptor-specific components. Regulator of chromosome condensation-2 (RCC2) was detected in the alpha(5)beta(1)-fibronectin signaling network at an intersection between the Rac1 and adenosine 5'-diphosphate ribosylation factor 6 (Arf6) subnetworks. RCC2 knockdown enhanced fibronectin-induced activation of both Rac1 and Arf6 and accelerated cell spreading, suggesting that RCC2 limits the signaling required for membrane protrusion and delivery. Dysregulation of Rac1 and Arf6 function by RCC2 knockdown also abolished persistent migration along fibronectin fibers, indicating a functional role for RCC2 in directional cell movement. This proteomics workflow now opens the way to further dissection and systems-level analyses of adhesion signaling.
Figures




References
-
- Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. - PubMed
-
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix and the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2001;2:793–805. - PubMed
-
- Delon I, Brown NH. Integrins and the actin cytoskeleton. Curr. Opin. Cell Biol. 2007;19:43–50. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

