An approach for reliably investigating hippocampal sharp wave-ripples in vitro
- PMID: 19738897
- PMCID: PMC2732900
- DOI: 10.1371/journal.pone.0006925
An approach for reliably investigating hippocampal sharp wave-ripples in vitro
Abstract
Background: Among the various hippocampal network patterns, sharp wave-ripples (SPW-R) are currently the mechanistically least understood. Although accurate information on synaptic interactions between the participating neurons is essential for comprehensive understanding of the network function during complex activities like SPW-R, such knowledge is currently notably scarce.
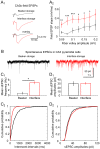
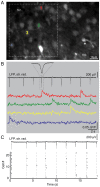
Methodology/principal findings: We demonstrate an in vitro approach to SPW-R that offers a simple experimental tool allowing detailed analysis of mechanisms governing the sharp wave-state of the hippocampus. We combine interface storage of slices with modifications of a conventional submerged recording system and established in vitro SPW-R comparable to their in vivo counterpart. We show that slice storage in the interface chamber close to physiological temperature is the required condition to preserve network integrity that is necessary for the generation of SPW-R. Moreover, we demonstrate the utility of our method for studying synaptic and network properties of SPW-R, using electrophysiological and imaging methods that can only be applied in the submerged system.
Conclusions/significance: The approach presented here demonstrates a reliable and experimentally simple strategy for studying hippocampal sharp wave-ripples. Given its utility and easy application we expect our model to foster the generation of new insight into the network physiology underlying SPW-R.
Conflict of interest statement
Figures





References
-
- Buzsáki G. Hippocampal sharp waves: their origin and significance. Brain Res. 1986;398:242–252. - PubMed
-
- Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. - PubMed
-
- Buzsáki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–570. - PubMed
-
- Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

