Role of plant glyoxylate reductases during stress: a hypothesis
- PMID: 19740079
- PMCID: PMC2762691
- DOI: 10.1042/BJ20090826
Role of plant glyoxylate reductases during stress: a hypothesis
Abstract
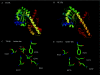
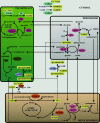
Molecular modelling suggests that a group of proteins in plants known as the beta-hydroxyacid dehydrogenases, or the hydroxyisobutyrate dehydrogenase superfamily, includes enzymes that reduce succinic semialdehyde and glyoxylate to gamma-hydroxybutyrate and glycolate respectively. Recent biochemical and expression studies reveal that NADPH-dependent cytosolic (termed GLYR1) and plastidial (termed GLYR2) isoforms of succinic semialdehyde/glyoxylate reductase exist in Arabidopsis. Succinic semialdehyde and glyoxylate are typically generated in leaves via two distinct metabolic pathways, gamma-aminobutyrate and glycolate respectively. In the present review, it is proposed that the GLYRs function in the detoxification of both aldehydes during stress and contribute to redox balance. Outstanding questions are highlighted in a scheme for the subcellular organization of the detoxification mechanism in Arabidopsis.
Figures

References
-
- Hoover G. J., Van Cauwenberghe O. R., Breitkreuz K. E., Clark S. M., Merrill A. R., Shelp B. J. Characteristics of an Arabidopsis glyoxylate reductase: general biochemical properties and substrate specificity for the recombinant protein, and developmental expression and implications for glyoxylate and succinic semialdehyde metabolism in planta. Can. J. Bot. 2007;85:883–895.
-
- Simpson J. P., Di Leo R., Dhanoa P. K., Allan W. L., Makhmoudova A., Clark S. M., Hoover G. J., Mullen R. T., Shelp B. J. Identification and characterization of a plastid-localized Arabidopsis glyoxylate reductase isoform: comparison with a cytosolic isoform and implications for cellular redox homeostasis and aldehyde detoxification. J. Exp. Bot. 2008;59:2454–2554. - PMC - PubMed
-
- Hoover G. J., Prentice G. A., Merrill A. R., Shelp B. J. Kinetic mechanism of an Arabidopsis glyoxylate reductase: studies of initial velocity, dead-end inhibition and product inhibition. Can. J. Bot. 2007;95:896–902.
-
- Weber H., Chetelat A., Reymond P., Farmer E. E. Selective and powerful stress gene expression in Arabidopsis in response to malondialdehyde. Plant J. 2004;37:877–888. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

