Hypoxia skews dendritic cells to a T helper type 2-stimulating phenotype and promotes tumour cell migration by dendritic cell-derived osteopontin
- PMID: 19740309
- PMCID: PMC2753916
- DOI: 10.1111/j.1365-2567.2008.02954.x
Hypoxia skews dendritic cells to a T helper type 2-stimulating phenotype and promotes tumour cell migration by dendritic cell-derived osteopontin
Abstract
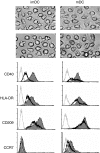
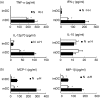
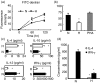
It is well recognized that tissue microenvironments are involved in regulating the development and function of dendritic cells (DC). Oxygen supply, which varies in different tissues, has been accepted as an important microenvironmental factor in regulating the biological functions of several immune cells and as being involved in tumour progression and metastasis. However, little is known about the effect of hypoxia on the biological functions of DC and the effect of these hypoxia-conditioned DC on tumour metastasis. In this study, we analysed the transcriptional profiles of human monocyte-derived immature DC (imDC) and mature DC (mDC) cultured under normoxia and hypoxia by microarray, and found a body of potential targets regulating the functions of DC during hypoxia. In addition, the phagocytic ability of hypoxic imDC markedly decreased compared with that of normoxic imDC. Importantly, hypoxic DC poorly induced the proliferation of allogeneic T cells, but polarized allogeneic CD4(+) naive T cells into a T helper type 2 (Th2) response. Moreover, hypoxic DC secreted large amounts of osteopontin, which were responsible for the enhanced migration of tumour cells. Therefore, our study provides new insights into the biological functions of DC under hypoxic conditions and one of mechanisms underlying tumour immune escape during hypoxia.
Figures
References
-
- Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG, Sitkovsky MV. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol. 2001;167:6140–9. - PubMed
-
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–65. - PubMed
-
- Brahimi-Horn MC, Pouyssegur J. The hypoxia-inducible factor and tumor progression along the angiogenic pathway. Int Rev Cytol. 2005;242:157–213. - PubMed
-
- Vaupel P, Briest S, Hockel M. Hypoxia in breast cancer: pathogenesis, characterization and biological/therapeutic implications. Wien Med Wochenschr. 2002;152:334–42. - PubMed
-
- Okunieff P, Fenton B, Chen Y. Past, present, and future of oxygen in cancer research. Adv Exp Med Biol. 2005;566:213–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

