Toll-like receptor agonists induce inflammation and cell death in a model of head and neck squamous cell carcinomas
- PMID: 19740321
- PMCID: PMC2753959
- DOI: 10.1111/j.1365-2567.2008.03041.x
Toll-like receptor agonists induce inflammation and cell death in a model of head and neck squamous cell carcinomas
Abstract
Toll-like receptors (TLRs) are increasingly implicated in the pathogenesis of cancer. The present study describes TLR expression and function in healthy and malignant airway epithelial cells. The squamous cell carcinoma cell line Detroit-562 was compared with the healthy bronchial epithelial cell line NL-20 and primary human nasal epithelial cells (HNECs). TLR2, TLR3 and TLR5 were present in primary head and neck squamous cell carcinomas (HNSCCs). Consistent with this, Detroit-562 expressed TLR2, TLR3 and TLR5, whereas NL-20 expressed mainly TLR3 and HNECs expressed TLR2-5. In Detroit-562, Pam(3)CSK(4), poly(I:C) and flagellin, ligands for TLR2, TLR3 and TLR5, respectively, induced an up-regulation of intercellular adhesion molecule 1 (ICAM-1), an increase in interleukin (IL)-6 and IL-8 secretion and a decrease in cell viability. Additionally, poly(I:C) affected IL-1beta production and the migratory behaviour of Detroit-562. NL-20 responded with a slight increase in IL-8 secretion upon poly(I:C) stimulation. Poly(I:C) induced a small increase in IL-1beta, IL-6 and IL-8 production in HNECs, while Pam(3)CSK(4) increased viability. The TLR signalling was transcription-dependent, but the pathways involved differed among TLRs as well as cells. In Detroit-562, TLR2 and TLR5 activation was mediated via c-jun N-terminal kinase (JNK)-, p38-, phosphatidylinositol 3-kinase (PI3K)- and nuclear factor (NF)-kappaB-related pathways, while TLR3 was dependent on NF-kappaB. In NL-20, TLR3 signalled via p38, and in HNECs, NF-kappaB, JNK and extracellular signal-regulated kinase (ERK) appeared to be involved. We found that TLR agonists induced a robust response in HNSCCs, characterized by generation of inflammation and cell death. A similar response was not seen in normal epithelial cells. Thus, the TLR system should be considered an important target in future antitumour immunotherapy.
Figures


 (n = 6). (c, d) Intracellular staining with monoclonal antibodies against TLRs (open histograms) or appropriate isotype controls (shaded histograms) in (c) Detroit-562 and (d) NL-20. Data show one representative out of three independent experiments.
(n = 6). (c, d) Intracellular staining with monoclonal antibodies against TLRs (open histograms) or appropriate isotype controls (shaded histograms) in (c) Detroit-562 and (d) NL-20. Data show one representative out of three independent experiments.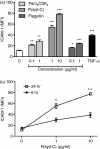




Similar articles
-
TLR2, TLR3 and TLR5 regulation of pro-inflammatory and pro-labour mediators in human primary myometrial cells.J Reprod Immunol. 2017 Aug;122:28-36. doi: 10.1016/j.jri.2017.08.004. Epub 2017 Aug 24. J Reprod Immunol. 2017. PMID: 28844021
-
Regulation of TLR2 expression and function in human airway epithelial cells.J Membr Biol. 2009 May;229(2):101-13. doi: 10.1007/s00232-009-9175-3. Epub 2009 Jun 10. J Membr Biol. 2009. PMID: 19513781
-
Poly(I:C) reduces expression of JAM-A and induces secretion of IL-8 and TNF-α via distinct NF-κB pathways in human nasal epithelial cells.Toxicol Appl Pharmacol. 2011 Jan 1;250(1):29-38. doi: 10.1016/j.taap.2010.09.023. Epub 2010 Oct 7. Toxicol Appl Pharmacol. 2011. PMID: 20932985
-
Roles of Toll-Like Receptor 3 in Human Tumors.Front Immunol. 2021 Apr 27;12:667454. doi: 10.3389/fimmu.2021.667454. eCollection 2021. Front Immunol. 2021. PMID: 33986756 Free PMC article. Review.
-
Inflammatory responses during trichomoniasis: The role of Toll-like receptors and inflammasomes.Parasite Immunol. 2023 Aug;45(8):e13000. doi: 10.1111/pim.13000. Epub 2023 Jun 20. Parasite Immunol. 2023. PMID: 37338019 Review.
Cited by
-
Antigen-presenting epithelial cells can play a pivotal role in airway allergy.J Allergy Clin Immunol. 2016 Mar;137(3):957-60.e7. doi: 10.1016/j.jaci.2015.08.053. Epub 2015 Nov 10. J Allergy Clin Immunol. 2016. PMID: 26560042 Free PMC article. No abstract available.
-
Magnetically attachable stencils and the non-destructive analysis of the contribution made by the underlying matrix to cell migration.Biomaterials. 2012 Nov;33(33):8189-203. doi: 10.1016/j.biomaterials.2012.07.018. Epub 2012 Aug 31. Biomaterials. 2012. PMID: 22940214 Free PMC article.
-
Crystalline silica-exposed human lung epithelial cells presented enhanced anchorage-independent growth with upregulated expression of BRD4 and EZH2 in autocrine and paracrine manners.PLoS One. 2023 May 5;18(5):e0285354. doi: 10.1371/journal.pone.0285354. eCollection 2023. PLoS One. 2023. PMID: 37146018 Free PMC article.
-
ICAM-1 orchestrates the abscopal effect of tumor radiotherapy.Proc Natl Acad Sci U S A. 2021 Apr 6;118(14):e2010333118. doi: 10.1073/pnas.2010333118. Proc Natl Acad Sci U S A. 2021. PMID: 33785590 Free PMC article.
-
Receptor-interacting protein kinase 1 is a key mediator in TLR3 ligand and Smac mimetic-induced cell death and suppresses TLR3 ligand-promoted invasion in cholangiocarcinoma.Cell Commun Signal. 2020 Oct 9;18(1):161. doi: 10.1186/s12964-020-00661-3. Cell Commun Signal. 2020. PMID: 33036630 Free PMC article.
References
-
- Greene CM, McElvaney NG. Toll-like receptor expression and function in airway epithelial cells. Arch Immunol Ther Exp (Warsz) 2005;53:418–27. - PubMed
-
- Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358–64. - PubMed
-
- Muir A, Soong G, Sokol S, Reddy B, Gomez MI, Van Heeckeren A, Prince A. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2004;30:777–83. - PubMed
-
- Tsan MF. Toll-like receptors, inflammation and cancer. Semin Cancer Biol. 2006;16:32–7. - PubMed
-
- Uematsu S, Akira S. Toll-like receptors and innate immunity. J Mol Med. 2006;84:712–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

