Granulocyte colony-stimulating factor-induced immature myeloid cells inhibit acute graft-versus-host disease lethality through an indoleamine dioxygenase-independent mechanism
- PMID: 19740324
- PMCID: PMC2753900
- DOI: 10.1111/j.1365-2567.2009.03048.x
Granulocyte colony-stimulating factor-induced immature myeloid cells inhibit acute graft-versus-host disease lethality through an indoleamine dioxygenase-independent mechanism
Abstract
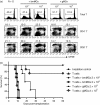
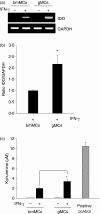
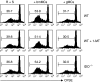
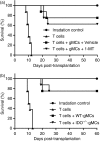
Granulocyte colony-stimulating factor (G-CSF)-mobilized donor graft tissue used for peripheral blood stem cell transplantation contains a large number of immature myeloid cells that suppress alloreactive donor T cells, resulting in an inhibition of acute graft-versus-host disease (GVHD). However, the molecular mechanism underlying the suppressive function of immature myeloid cells is not fully understood. Here, we investigated whether indoleamine 2,3-dioxygenase (IDO) is related to the suppressive mechanism of G-CSF-induced immature myeloid cells (gMCs). We found that Gr-1(+) CD11b(+) cells were highly induced in G-CSF-injected donor graft tissue, which is a phenotype of immature myeloid cells, resulting in an inhibition of acute GVHD lethality by suppressing alloreactive donor T-cell expansion. IDO was not detected in primary isolated gMCs; however, this enzyme was markedly induced after treatment with interferon-gamma (IFN-gamma). This level was significantly higher in IFN-gamma-treated gMCs than in bone marrow myeloid cells, which promote alloreactive T-cell responses. We next investigated the functional role of IDO in gMC-mediated inhibition of acute GVHD lethality. We found no changes in gMC-mediated survival or alloreactive donor T-cell suppression when IDO activity was blocked using 1-methyl tryptophan. In addition, there was no difference in gMC-mediated survival rates between recipients transferred with either wild-type gMCs or IDO(-/-) gMCs. Taken together, our data suggest that gMC-mediated inhibition of lethal acute GVHD is through an IDO-independent mechanism.
Figures

References
-
- Ringden O, Remberger M, Runde V, et al. Peripheral blood stem cell transplantation from unrelated donors: a comparison with marrow transplantation. Blood. 1999;94:455–64. - PubMed
-
- Miflin G, Russell NH, Hutchinson RM, et al. Allogeneic peripheral blood stem cell transplantation for haematological malignancies – an analysis of kinetics of engraftment and GVHD risk. Bone Marrow Transplant. 1997;19:9–13. - PubMed
-
- Ringden O, Remberger M, Runde V, et al. Faster engraftment of neutrophils and platelets with peripheral blood stem cells from unrelated donors: a comparison with marrow transplantation. Bone Marrow Transplant. 2000;25(Suppl. 2):S6–8. - PubMed
-
- Bensinger WI, Martin PJ, Storer B, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives inpatients with hematologic cancers. N Engl J Med. 2001;344:175–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

