Impact of human immunodeficiency virus 1 infection and inflammation on the composition and yield of cervical mononuclear cells in the female genital tract
- PMID: 19740336
- PMCID: PMC2753906
- DOI: 10.1111/j.1365-2567.2009.03077.x
Impact of human immunodeficiency virus 1 infection and inflammation on the composition and yield of cervical mononuclear cells in the female genital tract
Abstract
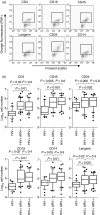
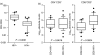
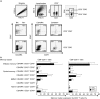
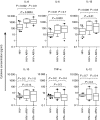
Cervical cytobrush sampling is a relatively non-invasive method for obtaining mucosal cells from the female genital tract. To define mucosal immune cells sampled by cervical cytobrushing and to validate this approach for local immunity studies, we investigated the impact of human immunodeficiency virus (HIV) status and inflammation on the yield and composition of cervical cytobrush specimens. Cervical cytobrush samples were obtained from 89 chronically HIV-infected and 46 HIV-negative women. The HIV-infected women had significantly higher yields of CD3(+), CD45(+), CD19(+), CD14(+), Langerin(+) and CD24(+) cells than the uninfected women. While cytobrush-derived T cells from uninfected women were predominantly CD4(+) (4.2 CD4 : 1 CD8), CD8(+) T cells were predominant in HIV-infected women (0.6 CD4 : 1 CD8). The majority of CD4(+) and CD8(+) T cells from HIV-infected and uninfected women were of the effector memory (CD45RA(-) CCR7(-) CD27(-)) phenotype. HIV-infected women had significantly elevated levels of interleukin (IL)-1beta, IL-6 and IL-8 in cervical supernatants compared with uninfected women. We observed a significant positive correlation between T-cell counts and IL-1beta, tumour necrosis factor (TNF)-alpha and IL-12 concentrations. Neutrophil counts correlated significantly with cervical concentrations of IL-1beta, TNF-alpha, IL-8, IL-6 and IL-10. Antigen-presenting cell numbers correlated significantly with TNF-alpha and IL-12 concentrations. HIV-infected women on antiretroviral therapy had similar levels of cervical lymphocyte infiltration and inflammation to women naïve to therapy. In conclusion, we suggest that inflammation at the cervix and HIV infection are likely to be key determinants in the absolute number of mucosal immune cells recovered by cervical cytobrushing.
Figures
References
-
- UNAIDS . AIDS epidemic update. 2007. URL http://data.unaids.org/pub/FactSheet/2008/(epi07_fs_regionalsummary_subs... [accessed on 08 April 2009]
-
- Anton PA, Elliott J, Poles MA, et al. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS. 2000;14:1761–1765. - PubMed
-
- Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

