Local irradiation of murine melanoma affects the development of tumour-specific immunity
- PMID: 19740341
- PMCID: PMC2753951
- DOI: 10.1111/j.1365-2567.2009.03084.x
Local irradiation of murine melanoma affects the development of tumour-specific immunity
Abstract
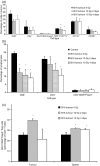
Radiation therapy affects the immune system. In addition to killing radiosensitive immune cells, it can induce functional changes in those cells that survive. Our recent studies showed that the exposure of dendritic cells (DCs) to radiation in vitro influences their ability to present tumour antigen in vivo. Here we show that local radiation therapy of B16 melanoma tumours inhibits the development of systemic immunity to the melanoma antigen MART-1. This inhibition could not be overcome by intratumoral injection of DCs expressing human MART-1 after radiation therapy, suggesting that a form of immune suppression might have developed. On the other hand, injection of MART-expressing DCs prior to tumour irradiation was able to prevent inhibition from developing. These results suggest that local radiation therapy may block the generation of immunity under some circumstances and that strategies may be required to prevent this and allow radiation-induced cell death to translate fully into the development of systemic immunity.
Figures

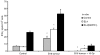


Similar articles
-
Ionizing radiation affects human MART-1 melanoma antigen processing and presentation by dendritic cells.J Immunol. 2004 Aug 15;173(4):2462-9. doi: 10.4049/jimmunol.173.4.2462. J Immunol. 2004. PMID: 15294960
-
Mechanisms involved in radiation enhancement of intratumoral dendritic cell therapy.J Immunother. 2008 May;31(4):345-58. doi: 10.1097/CJI.0b013e318163628c. J Immunother. 2008. PMID: 18391761 Free PMC article.
-
Generation of T-cell immunity to a murine melanoma using MART-1-engineered dendritic cells.J Immunother. 2000 Jan;23(1):59-66. doi: 10.1097/00002371-200001000-00008. J Immunother. 2000. PMID: 10687138
-
Review: dendritic cell immunotherapy for melanoma.Cancer Biother Radiopharm. 1999 Feb;14(1):11-22. doi: 10.1089/cbr.1999.14.11. Cancer Biother Radiopharm. 1999. PMID: 10850282 Review.
-
Pulsing of dendritic cells with cell lysates from either B16 melanoma or MCA-106 fibrosarcoma yields equally effective vaccines against B16 tumors in mice.J Surg Oncol. 1998 Jun;68(2):79-91. doi: 10.1002/(sici)1096-9098(199806)68:2<79::aid-jso3>3.0.co;2-h. J Surg Oncol. 1998. PMID: 9624036 Review.
Cited by
-
The confluence of stereotactic ablative radiotherapy and tumor immunology.Clin Dev Immunol. 2011;2011:439752. doi: 10.1155/2011/439752. Epub 2011 Nov 15. Clin Dev Immunol. 2011. PMID: 22162711 Free PMC article. Review.
-
Feasibility study of DCs/CIKs combined with thoracic radiotherapy for patients with locally advanced or metastatic non-small-cell lung cancer.Radiat Oncol. 2016 Apr 21;11:60. doi: 10.1186/s13014-016-0635-5. Radiat Oncol. 2016. PMID: 27097970 Free PMC article. Clinical Trial.
-
Modern Radiotherapy Concepts and the Impact of Radiation on Immune Activation.Front Oncol. 2016 Jun 20;6:141. doi: 10.3389/fonc.2016.00141. eCollection 2016. Front Oncol. 2016. PMID: 27379203 Free PMC article. Review.
-
Targeting the inhibitory receptor CTLA-4 on T cells increased abscopal effects in murine mesothelioma model.Oncotarget. 2015 May 20;6(14):12468-80. doi: 10.18632/oncotarget.3487. Oncotarget. 2015. PMID: 25980578 Free PMC article.
-
The evolution of rectal and urinary toxicity and immune response in prostate cancer patients treated with two three-dimensional conformal radiotherapy techniques.Radiat Oncol. 2011 Jul 27;6:87. doi: 10.1186/1748-717X-6-87. Radiat Oncol. 2011. PMID: 21794152 Free PMC article.
References
-
- Williams JL, Patchen ML, Darden JH, Jackson WE. Effects of radiation on survival and recovery of T lymphocyte subsets in C3H/HeN mice. Exp Hematol. 1994;22:510–6. - PubMed
-
- Anderson RE, Warner NL. Ionizing radiation and the immune response. Adv Immunol. 1976;24:215–335. - PubMed
-
- Chang CM, Limanni A, Baker WH, Dobson ME, Kalinich JF, Patchen ML. Sublethal gamma irradiation increases IL-1alpha, IL-6, and TNF-alpha mRNA levels in murine hematopoietic tissues. J Interferon Cytokine Res. 1997;17:567–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

