Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model
- PMID: 19740383
- PMCID: PMC2767316
- DOI: 10.1111/j.1365-2567.2009.03115.x
Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model
Abstract
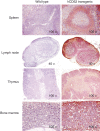
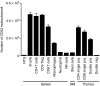
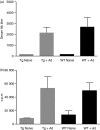
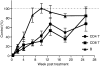
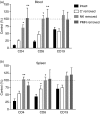
Alemtuzumab is a humanized monoclonal antibody against CD52, an antigen found on the surface of normal and malignant lymphocytes. It is approved for the treatment of B-cell chronic lymphocytic leukaemia and is undergoing Phase III clinical trials for the treatment of multiple sclerosis. The exact mechanism by which alemtuzumab mediates its biological effects in vivo is not clearly defined and mechanism of action studies have been hampered by the lack of cross-reactivity between human and mouse CD52. To address this issue, a transgenic mouse expressing human CD52 (hCD52) was created. Transgenic mice did not display any phenotypic abnormalities and were able to mount normal immune responses. The tissue distribution of hCD52 and the level of expression by various immune cell populations were comparable to those seen in humans. Treatment with alemtuzumab replicated the transient increase in serum cytokines and depletion of peripheral blood lymphocytes observed in humans. Lymphocyte depletion was not as profound in lymphoid organs, providing a possible explanation for the relatively low incidence of infection in alemtuzumab-treated patients. Interestingly, both lymphocyte depletion and cytokine induction by alemtuzumab were largely independent of complement and appeared to be mediated by neutrophils and natural killer cells because removal of these populations with antibodies to Gr-1 or asialo-GM-1, respectively, strongly inhibited the activity of alemtuzumab whereas removal of complement by treatment with cobra venom factor had no impact. The hCD52 transgenic mouse appears to be a useful model and has provided evidence for the previously uncharacterized involvement of neutrophils in the activity of alemtuzumab.
Figures


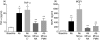
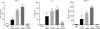
References
-
- Hale G, Xia MQ, Tighe HP, Dyer MJ, Waldmann H. The CAMPATH-1antigen (CDw52) Tissue Antigens. 1990;35:118–27. - PubMed
-
- Hale G. CD52 (Campath-1) J Biol Regul Homeost Agents. 2001;15:386–91. - PubMed
-
- Huh Y, Kantarjian H, Pierce SM, et al. Expression of human CD52 in human hematopoietic malignancies. Blood. 1998;92 Abstract 4199.
-
- Elsner J, Hochstetter R, Spiekermann K, Kapp A. Surface and mRNA expression of the CD52 antigen by human eosinophils but not by neutrophils. Blood. 1996;88:4684–93. - PubMed
-
- Gilleece MH, Dexter TM. Effect of Campath-1H antibody on human hematopoietic progenitors in vitro. Blood. 1993;82:807–12. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

