Beta(2) integrin deficiency yields unconventional double-negative T cells distinct from mature classical natural killer T cells in mice
- PMID: 19740384
- PMCID: PMC2767317
- DOI: 10.1111/j.1365-2567.2009.03116.x
Beta(2) integrin deficiency yields unconventional double-negative T cells distinct from mature classical natural killer T cells in mice
Abstract
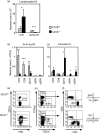
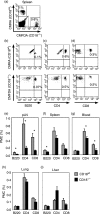
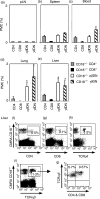
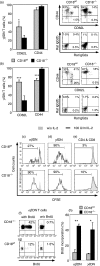
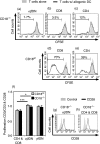
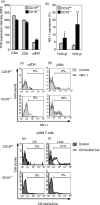
Expressed on leucocytes, beta(2) integrins (CD11/CD18) are specifically involved in leucocyte function. Using a CD18-deficient (CD18(-/-)) mouse model, we here report on their physiological role in lymphocyte differentiation and trafficking. CD18(-/-) mice present with a defect in the distribution of lymphocytes with highly reduced numbers of naïve B and T lymphocytes in inguinal and axillary lymph nodes. In contrast, cervical lymph nodes were fourfold enlarged harbouring unconventional T-cell receptor-alphabeta (TCR-alphabeta) and TCR-gammadelta CD3(+) CD4(-) CD8(-) (double-negative; DN) T cells that expanded in situ. Using adoptive transfer experiments, we found that these cells did not home to peripheral lymph nodes of CD18(wt) recipients but, like antigen-experienced T or natural killer (NK) T cells, recirculated through non-lymphoid organs. Lacking regulatory functions in vitro, CD18(-/-) TCR-alphabeta DN T cells did not suppress the proliferation of polyclonally activated CD4(+) or CD8(+) (single-positive; SP) T cells. Most interestingly, CD18(-/-) TCR-alphabeta DN T cells showed intermediate TCR expression levels, an absent activation through allogeneic major histocompatibility complex and a strong proliferative dependence on interleukin-2, hence, closely resembling NKT cells. However, our data oppose former reports, clearly showing that, because of an absent reactivity with CD1d-alphaGalCer dimers, these cells are not mature classical NKT cells. Our data indicate that CD18(-/-) TCR-alphabeta DN T cells, like NKT and TCR-gammadelta T cells, share characteristics of both adaptive and innate immune cells, and may accumulate as a compensatory mechanism to the functional defect of adaptive immunity in CD18(-/-) mice.
Figures

Similar articles
-
MicroRNA-181a/b-1 Is Not Required for Innate γδ NKT Effector Cell Development.PLoS One. 2015 Dec 16;10(12):e0145010. doi: 10.1371/journal.pone.0145010. eCollection 2015. PLoS One. 2015. PMID: 26673421 Free PMC article.
-
Defining a novel subset of CD1d-dependent type II natural killer T cells using natural killer cell-associated markers.Scand J Immunol. 2019 Sep;90(3):e12794. doi: 10.1111/sji.12794. Epub 2019 Jun 26. Scand J Immunol. 2019. PMID: 31141185 Free PMC article.
-
Development of CD4-CD8- alpha beta TCR+NK1.1+ T lymphocytes: thymic selection by self antigen.J Immunol. 1996 Nov 15;157(10):4379-89. J Immunol. 1996. PMID: 8906813
-
Porcine T lymphocytes and NK cells--an update.Dev Comp Immunol. 2009 Mar;33(3):310-20. doi: 10.1016/j.dci.2008.06.003. Epub 2008 Jul 2. Dev Comp Immunol. 2009. PMID: 18601948 Review.
-
The Role of Adaptor Proteins in the Biology of Natural Killer T (NKT) Cells.Front Immunol. 2019 Jun 25;10:1449. doi: 10.3389/fimmu.2019.01449. eCollection 2019. Front Immunol. 2019. PMID: 31293596 Free PMC article. Review.
Cited by
-
The integrin adhesome: from genes and proteins to human disease.Nat Rev Mol Cell Biol. 2014 Apr;15(4):273-88. doi: 10.1038/nrm3769. Nat Rev Mol Cell Biol. 2014. PMID: 24651544 Review.
-
β2 Integrins differentially regulate γδ T cell subset thymic development and peripheral maintenance.Proc Natl Acad Sci U S A. 2020 Sep 8;117(36):22367-22377. doi: 10.1073/pnas.1921930117. Epub 2020 Aug 26. Proc Natl Acad Sci U S A. 2020. PMID: 32848068 Free PMC article.
-
Adaptive immune response to model antigens is impaired in murine leukocyte-adhesion deficiency-1 revealing elevated activation thresholds in vivo.Clin Dev Immunol. 2012;2012:450738. doi: 10.1155/2012/450738. Epub 2012 Mar 4. Clin Dev Immunol. 2012. PMID: 22474478 Free PMC article.
-
Hematopoietic stem/progenitor cells directly contribute to arteriosclerotic progression via integrin β2.Stem Cells. 2015 Apr;33(4):1230-40. doi: 10.1002/stem.1939. Stem Cells. 2015. PMID: 25546260 Free PMC article.
-
T cell-extrinsic CD18 attenuates antigen-dependent CD4+ T cell activation in vivo.J Immunol. 2015 May 1;194(9):4122-9. doi: 10.4049/jimmunol.1401328. Epub 2015 Mar 23. J Immunol. 2015. PMID: 25801431 Free PMC article.
References
-
- Yong K, Khwaja A. Leucocyte cellular adhesion molecules. Blood Rev. 1990;4:211–25. - PubMed
-
- Arnaout MA. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990;75:1037–50. - PubMed
-
- Hamann A, Jablonski-Westrich D, Duijvestijn A, Butcher EC, Baisch H, Harder R, Thiele HG. Evidence for an accessory role of LFA-1 in lymphocyte-high endothelium interaction during homing. J Immunol. 1988;140:693–9. - PubMed
-
- Anderson DC, Springer TA. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

