Nebulized antithrombin limits bacterial outgrowth and lung injury in Streptococcus pneumoniae pneumonia in rats
- PMID: 19740417
- PMCID: PMC2784363
- DOI: 10.1186/cc8040
Nebulized antithrombin limits bacterial outgrowth and lung injury in Streptococcus pneumoniae pneumonia in rats
Abstract
Introduction: Disturbed alveolar fibrin turnover is a cardinal feature of severe pneumonia. Clinical studies suggest that natural inhibitors of coagulation exert lung-protective effects via anticoagulant and possibly also anti-inflammatory pathways. Intravenous infusion of the natural anticoagulants increases the risk of bleeding. Local administration may allow for higher treatment dosages and increased local efficacy while at the same time reducing the risk of bleeding. We evaluated the effect of nebulized anticoagulants on pulmonary coagulopathy and inflammation in a rat model of Streptococcus pneumoniae pneumonia.
Methods: In this randomized controlled in vivo laboratory study rats were challenged intratracheally with S. pneumoniae, inducing pneumonia, and randomized to treatment with normal saline (placebo), recombinant human activated protein C (rh-APC), plasma-derived antithrombin (AT), heparin or danaparoid, by means of nebulization.
Results: S. pneumoniae infection increased pulmonary levels of thrombin-antithrombin complexes and fibrin degradation products. All nebulized anticoagulants significantly limited pulmonary coagulopathy. None of the agents except danaparoid resulted in changes in systemic coagulopathy. Treatment with plasma-derived AT reduced outgrowth of S. pneumoniae and histopathologic damage in lungs. In vitro experiments confirmed outgrowth was reduced in bronchoalveolar lavage fluid (BALF) from rats treated with plasma-derived AT compared with placebo. Neutralizing of cationic components in BALF diminished the inhibitory effects on bacterial outgrowth of BALF, suggesting a role for cationic antimicrobial proteins.
Conclusions: Nebulization of anticoagulants attenuates pulmonary coagulopathy during S. pneumoniae pneumonia in rats while only danaparoid affects systemic coagulation. Nebulized plasma-derived AT reduces bacterial outgrowth and exerts significant lung-protective effects.
Figures

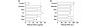
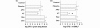
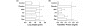



Comment in
-
Direct antimicrobial activity of antithrombin?Crit Care. 2010;14(5):440. doi: 10.1186/cc9243. Epub 2010 Sep 7. Crit Care. 2010. PMID: 20854649 Free PMC article. No abstract available.
Similar articles
-
Nebulized anticoagulants limit coagulopathy but not inflammation in pseudomonas aeruginosa-induced pneumonia in rats.Shock. 2011 Oct;36(4):417-23. doi: 10.1097/SHK.0b013e31822bcef0. Shock. 2011. PMID: 21897338
-
Antithrombin inhibits bronchoalveolar activation of coagulation and limits lung injury during Streptococcus pneumoniae pneumonia in rats.Crit Care Med. 2008 Jan;36(1):204-10. doi: 10.1097/01.CCM.0000292012.87482.F4. Crit Care Med. 2008. PMID: 18090375
-
Plasma-derived human antithrombin attenuates ventilator-induced coagulopathy but not inflammation in a Streptococcus pneumoniae pneumonia model in rats.J Thromb Haemost. 2012 Mar;10(3):399-410. doi: 10.1111/j.1538-7836.2012.04622.x. J Thromb Haemost. 2012. PMID: 22236057
-
Nebulized anticoagulants in lung injury in critically ill patients-an updated systematic review of preclinical and clinical studies.Ann Transl Med. 2017 Nov;5(22):444. doi: 10.21037/atm.2017.08.23. Ann Transl Med. 2017. PMID: 29264361 Free PMC article. Review.
-
Bronchoalveolar hemostasis in lung injury and acute respiratory distress syndrome.J Thromb Haemost. 2013 Jan;11(1):17-25. doi: 10.1111/jth.12047. J Thromb Haemost. 2013. PMID: 23114008 Review.
Cited by
-
"War to the knife" against thromboinflammation to protect endothelial function of COVID-19 patients.Crit Care. 2020 Jun 19;24(1):365. doi: 10.1186/s13054-020-03060-9. Crit Care. 2020. PMID: 32560665 Free PMC article.
-
Direct antimicrobial activity of antithrombin?Crit Care. 2010;14(5):440. doi: 10.1186/cc9243. Epub 2010 Sep 7. Crit Care. 2010. PMID: 20854649 Free PMC article. No abstract available.
-
Effects of nebulized antithrombin and heparin on inflammatory and coagulation alterations in an acute lung injury model in rats.J Thromb Haemost. 2020 Mar;18(3):571-583. doi: 10.1111/jth.14685. Epub 2019 Dec 16. J Thromb Haemost. 2020. PMID: 31755229 Free PMC article.
-
Nebulized anticoagulants for acute lung injury - a systematic review of preclinical and clinical investigations.Crit Care. 2012 Dec 12;16(2):R70. doi: 10.1186/cc11325. Crit Care. 2012. PMID: 22546487 Free PMC article.
-
Serine Protease Inhibitors Restrict Host Susceptibility to SARS-CoV-2 Infections.mBio. 2022 Jun 28;13(3):e0089222. doi: 10.1128/mbio.00892-22. Epub 2022 May 9. mBio. 2022. PMID: 35532162 Free PMC article.
References
-
- Choi G, Hofstra JJ, Roelofs JJ, Rijneveld AW, Bresser P, Zee JS van der, Florquin S, Poll T van der, Levi M, Schultz MJ. Antithrombin inhibits bronchoalveolar activation of coagulation and limits lung injury during Streptococcus pneumoniae pneumonia in rats. Crit Care Med. 2008;36:204–210. doi: 10.1097/01.CCM.0000292012.87482.F4. - DOI - PubMed
-
- Gunther A, Mosavi P, Heinemann S, Ruppert C, Muth H, Markart P, Grimminger F, Walmrath D, Temmesfeld-Wollbrück B, Seeger W. Alveolar fibrin formation caused by enhanced procoagulant and depressed fibrinolytic capacities in severe pneumonia. Comparison with the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161:454–462. - PubMed
-
- Rijneveld AW, Weijer S, Bresser P, Florquin S, Vlasuk GP, Rote WE, Spek CA, Reitsma PH, Zee JS van der, Levi M, Poll T van der. Local activation of the tissue factor-factor VIIa pathway in patients with pneumonia and the effect of inhibition of this pathway in murine pneumococcal pneumonia. Crit Care Med. 2006;34:1725–1730. doi: 10.1097/01.CCM.0000218807.20570.C2. - DOI - PubMed
-
- Idell S, James KK, Levin EG, Schwartz BS, Manchanda N, Maunder RJ, Martin TR, McLarty J, Fair DS. Local abnormalities in coagulation and fibrinolytic pathways predispose to alveolar fibrin deposition in the adult respiratory distress syndrome. J Clin Invest. 1989;84:695–705. doi: 10.1172/JCI114217. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

