Efficacy and safety of tigecycline versus levofloxacin for community-acquired pneumonia
- PMID: 19740418
- PMCID: PMC2753558
- DOI: 10.1186/1471-2466-9-44
Efficacy and safety of tigecycline versus levofloxacin for community-acquired pneumonia
Abstract
Background: Tigecycline, an expanded broad-spectrum glycylcycline, exhibits in vitro activity against many common pathogens associated with community-acquired pneumonia (CAP), as well as penetration into lung tissues that suggests effectiveness in hospitalized CAP patients. The aim of the present study was to compare the efficacy and safety of intravenous (IV) tigecycline with IV levofloxacin in hospitalized adults with CAP.
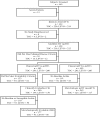
Methods: In this prospective, double-blind, non-inferiority phase 3 trial, eligible patients with a clinical diagnosis of CAP supported by radiographic evidence were stratified by Fine Pneumonia Severity Index and randomized to tigecycline or levofloxacin for 7-14 days of therapy. Co-primary efficacy endpoints were clinical response in the clinically evaluable (CE) and clinical modified intent-to-treat (c-mITT) populations at test-of-cure (Day 10-21 post-therapy).
Results: Of the 428 patients who received at least one dose of study drug, 79% had CAP of mild-moderate severity according to their Fine score. Clinical cure rates for the CE population were 88.9% for tigecycline and 85.3% for levofloxacin. Corresponding c-mITT population rates were 83.7% and 81.5%, respectively. Eradication rates for Streptococcus pneumoniae were 92% for tigecycline and 89% for levofloxacin. Nausea, vomiting, and diarrhoea were the most frequently reported adverse events. Rates of premature discontinuation of study drug or study withdrawal because of any adverse event were similar for both study drugs.
Conclusion: These findings suggest that IV tigecycline is non-inferior to IV levofloxacin and is generally well-tolerated in the treatment of hospitalized adults with CAP.
Trial registration: NCT00081575.
Figures
References
-
- Aleva RM, Boersma WG. [Guideline 'Diagnosis and treatment of community-acquired pneumonia' from the Dutch Thoracic Society] Ned Tijdschr Geneeskd. 2005;149:2501–2507. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

