A novel biosynthetic pathway of archaetidyl-myo-inositol via archaetidyl-myo-inositol phosphate from CDP-archaeol and D-glucose 6-phosphate in methanoarchaeon Methanothermobacter thermautotrophicus cells
- PMID: 19740749
- PMCID: PMC2781475
- DOI: 10.1074/jbc.M109.034652
A novel biosynthetic pathway of archaetidyl-myo-inositol via archaetidyl-myo-inositol phosphate from CDP-archaeol and D-glucose 6-phosphate in methanoarchaeon Methanothermobacter thermautotrophicus cells
Abstract
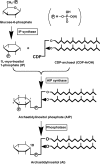
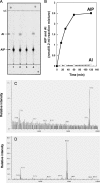
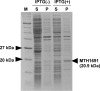
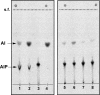
Ether-type inositol phospholipids are ubiquitously distributed in Archaea membranes. The present paper describes a novel biosynthetic pathway of the archaeal inositol phospholipid. To study the biosynthesis of archaetidylinositol in vitro, we prepared two possible substrates: CDP-archaeol, which was chemically synthesized, and myo-[(14)C]inositol 1-phosphate, which was enzymatically prepared from [(14)C]glucose 6-phosphate with the inositol 1-phosphate (IP) synthase of this organism. The complete structure of the IP synthase reaction product was determined to be 1l-myo-inositol 1-phosphate, based on gas liquid chromatography with a chiral column. When the two substrates were incubated with the Methanothermobacter thermautotrophicus membrane fraction, archaetidylinositol phosphate (AIP) was formed along with a small amount of archaetidylinositol (AI). The two products were identified by fast atom bombardment-mass spectrometry and chemical analyses. AI was formed from AIP by incubation with the membrane fraction, but AIP was not formed from AI. This finding indicates that archaeal AI was synthesized from CDP-archaeol and d-glucose 6-phosphate via myo-inositol 1-phosphate and AIP. Although the relevant enzymes were not isolated, three enzymes are implied: IP synthase, AIP synthase, and AIP phosphatase. AIP synthase was homologous to yeast phosphatidylinositol synthase, and we confirmed AIP synthase activity by cloning the encoding gene (MTH1691) and expressing it in Escherichia coli. AIP synthase is a newly found member of the enzyme superfamily CDP-alcohol phosphatidyltransferase, which includes a wide range of enzymes that attach polar head groups to ester- and ether-type phospholipids of bacterial and archaeal origin. This is the first report of the biosynthesis of ether-type inositol phospholipids in Archaea.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

