Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein
- PMID: 19740980
- PMCID: PMC2772693
- DOI: 10.1128/JVI.01515-09
Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein
Abstract
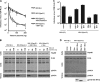
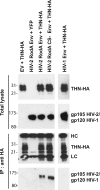
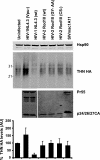
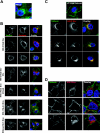
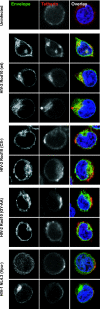
Tetherin (CD317/BST-2), an interferon-induced membrane protein, restricts the release of nascent retroviral particles from infected cell surfaces. While human immunodeficiency virus type 1 (HIV-1) encodes the accessory gene vpu to overcome the action of tetherin, the lineage of primate lentiviruses that gave rise to HIV-2 does not. It has been previously reported that the HIV-2 envelope glycoprotein has a Vpu-like function in promoting virus release. Here we demonstrate that the HIV-2 Rod envelope glycoprotein (HIV-2 Rod Env) is a tetherin antagonist. Expression of HIV-2 Rod Env, but not that of HIV-1 or the closely related simian immunodeficiency virus (SIV) SIVmac1A11, counteracts tetherin-mediated restriction of Vpu-defective HIV-1 in a cell-type-specific manner. This correlates with the ability of the HIV-2 Rod Env to mediate cell surface downregulation of tetherin. Antagonism requires an endocytic motif conserved across HIV/SIV lineages in the gp41 cytoplasmic tail, but specificity for tetherin is governed by extracellular determinants in the mature Env protein. Coimmunoprecipitation studies suggest an interaction between HIV-2 Rod Env and tetherin, but unlike studies with Vpu, we found no evidence of tetherin degradation. In the presence of HIV-2 Rod Env, tetherin localization is restricted to the trans-Golgi network, suggesting Env-mediated effects on tetherin trafficking sequester it from virus assembly sites on the plasma membrane. Finally, we recapitulated these observations in HIV-2-infected CD4+ T-cell lines, demonstrating that tetherin antagonism and sequestration occur at physiological levels of Env expression during virus replication.
Figures





References
-
- Boge, M., S. Wyss, J. S. Bonifacino, and M. Thali. 1998. A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J. Biol. Chem. 273:15773-15778. - PubMed
-
- Bour, S., H. Akari, E. Miyagi, and K. Strebel. 2003. Naturally occurring amino acid substitutions in the HIV-2 ROD envelope glycoprotein regulate its ability to augment viral particle release. Virology 309:85-98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

