GBF1, a guanine nucleotide exchange factor for Arf, is crucial for coxsackievirus B3 RNA replication
- PMID: 19740986
- PMCID: PMC2772713
- DOI: 10.1128/JVI.01244-09
GBF1, a guanine nucleotide exchange factor for Arf, is crucial for coxsackievirus B3 RNA replication
Abstract
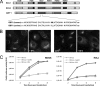
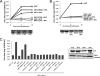
The replication of enteroviruses is sensitive to brefeldin A (BFA), an inhibitor of endoplasmic reticulum-to-Golgi network transport that blocks activation of guanine exchange factors (GEFs) of the Arf GTPases. Mammalian cells contain three BFA-sensitive Arf GEFs: GBF1, BIG1, and BIG2. Here, we show that coxsackievirus B3 (CVB3) RNA replication is insensitive to BFA in MDCK cells, which contain a BFA-resistant GBF1 due to mutation M832L. Further evidence for a critical role of GBF1 stems from the observations that viral RNA replication is inhibited upon knockdown of GBF1 by RNA interference and that replication in the presence of BFA is rescued upon overexpression of active, but not inactive, GBF1. Overexpression of Arf proteins or Rab1B, a GTPase that induces GBF1 recruitment to membranes, failed to rescue RNA replication in the presence of BFA. Additionally, the importance of the interaction between enterovirus protein 3A and GBF1 for viral RNA replication was investigated. For this, the rescue from BFA inhibition of wild-type (wt) replicons and that of mutant replicons of both CVB3 and poliovirus (PV) carrying a 3A protein that is impaired in binding GBF1 were compared. The BFA-resistant GBF1-M832L protein efficiently rescued RNA replication of both wt and mutant CVB3 and PV replicons in the presence of BFA. However, another BFA-resistant GBF1 protein, GBF1-A795E, also efficiently rescued RNA replication of the wt replicons, but not that of mutant replicons, in the presence of BFA. In conclusion, this study identifies a critical role for GBF1 in CVB3 RNA replication, but the importance of the 3A-GBF1 interaction requires further study.
Figures




Similar articles
-
The development of resistance to an inhibitor of a cellular protein reveals a critical interaction between the enterovirus protein 2C and a small GTPase Arf1.PLoS Pathog. 2023 Sep 18;19(9):e1011673. doi: 10.1371/journal.ppat.1011673. eCollection 2023 Sep. PLoS Pathog. 2023. PMID: 37721955 Free PMC article.
-
Recruitment of PI4KIIIβ to coxsackievirus B3 replication organelles is independent of ACBD3, GBF1, and Arf1.J Virol. 2014 Mar;88(5):2725-36. doi: 10.1128/JVI.03650-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352456 Free PMC article.
-
Differential effects of the putative GBF1 inhibitors Golgicide A and AG1478 on enterovirus replication.J Virol. 2010 Aug;84(15):7535-42. doi: 10.1128/JVI.02684-09. Epub 2010 May 26. J Virol. 2010. PMID: 20504936 Free PMC article.
-
Role of the Guanine Nucleotide Exchange Factor GBF1 in the Replication of RNA Viruses.Viruses. 2020 Jun 24;12(6):682. doi: 10.3390/v12060682. Viruses. 2020. PMID: 32599855 Free PMC article. Review.
-
Regulating the regulators: role of phosphorylation in modulating the function of the GBF1/BIG family of Sec7 ARF-GEFs.FEBS Lett. 2020 Jul;594(14):2213-2226. doi: 10.1002/1873-3468.13798. Epub 2020 May 14. FEBS Lett. 2020. PMID: 32333796 Review.
Cited by
-
Recent progress in understanding coxsackievirus replication, dissemination, and pathogenesis.Virology. 2015 Oct;484:288-304. doi: 10.1016/j.virol.2015.06.006. Epub 2015 Jul 1. Virology. 2015. PMID: 26142496 Free PMC article. Review.
-
A Proximity biotinylation assay with a host protein bait reveals multiple factors modulating enterovirus replication.PLoS Pathog. 2022 Oct 28;18(10):e1010906. doi: 10.1371/journal.ppat.1010906. eCollection 2022 Oct. PLoS Pathog. 2022. PMID: 36306280 Free PMC article.
-
The development of resistance to an inhibitor of a cellular protein reveals a critical interaction between the enterovirus protein 2C and a small GTPase Arf1.PLoS Pathog. 2023 Sep 18;19(9):e1011673. doi: 10.1371/journal.ppat.1011673. eCollection 2023 Sep. PLoS Pathog. 2023. PMID: 37721955 Free PMC article.
-
Golgi bypass: skirting around the heart of classical secretion.Cold Spring Harb Perspect Biol. 2011 Apr 1;3(4):a005298. doi: 10.1101/cshperspect.a005298. Cold Spring Harb Perspect Biol. 2011. PMID: 21441587 Free PMC article. Review.
-
Direct-Acting Antivirals and Host-Targeting Approaches against Enterovirus B Infections: Recent Advances.Pharmaceuticals (Basel). 2023 Jan 29;16(2):203. doi: 10.3390/ph16020203. Pharmaceuticals (Basel). 2023. PMID: 37259352 Free PMC article. Review.
References
-
- Aminev, A. G., S. P. Amineva, and A. C. Palmenberg. 2003. Encephalomyocarditis virus (EMCV) proteins 2A and 3BCD localize to nuclei and inhibit cellular mRNA transcription but not rRNA transcription. Virus Res. 95:59-73. - PubMed
-
- Balch, W. E., R. A. Kahn, and R. Schwaninger. 1992. ADP-ribosylation factor is required for vesicular trafficking between the endoplasmic reticulum and the cis-Golgi compartment. J. Biol. Chem. 267:13053-13061. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

