HOPS interacts with Apl5 at the vacuole membrane and is required for consumption of AP-3 transport vesicles
- PMID: 19741093
- PMCID: PMC2770944
- DOI: 10.1091/mbc.e09-04-0272
HOPS interacts with Apl5 at the vacuole membrane and is required for consumption of AP-3 transport vesicles
Abstract
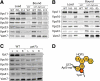
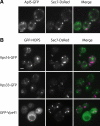
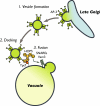
Adaptor protein complexes (APs) are evolutionarily conserved heterotetramers that couple cargo selection to the formation of highly curved membranes during vesicle budding. In Saccharomyces cerevisiae, AP-3 mediates vesicle traffic from the late Golgi to the vacuolar lysosome. The HOPS subunit Vps41 is one of the few proteins reported to have a specific role in AP-3 traffic, yet its function remains undefined. We now show that although the AP-3 delta subunit, Apl5, binds Vps41 directly, this interaction occurs preferentially within the context of the HOPS docking complex. Fluorescence microscopy indicates that Vps41 and other HOPS subunits do not detectably colocalize with AP-3 at the late Golgi or on post-Golgi (Sec7-negative) vesicles. Vps41 and HOPS do, however, transiently colocalize with AP-3 vesicles when these vesicles dock at the vacuole membrane. In cells with mutations in HOPS subunits or the vacuole SNARE Vam3, AP-3 shifts from the cytosol to a membrane fraction. Fluorescence microscopy suggests that this fraction consists of post-Golgi AP-3 vesicles that have failed to dock or fuse at the vacuole membrane. We propose that AP-3 remains associated with budded vesicles, interacts with Vps41 and HOPS upon vesicle docking at the vacuole, and finally dissociates during docking or fusion.
Figures






References
-
- Baggett J. J., Wendland B. Clathrin function in yeast endocytosis. Traffic. 2001;2:297–302. - PubMed
-
- Bonifacino J. S., Glick B. S. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. - PubMed
-
- Cai H., Yu S., Menon S., Cai Y., Lazarova D., Fu C., Reinisch K., Hay J. C., Ferro-Novick S. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature. 2007;445:941–944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

