Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers
- PMID: 19741128
- PMCID: PMC2771749
- DOI: 10.1523/JNEUROSCI.6096-08.2009
Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers
Abstract
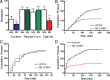
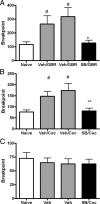
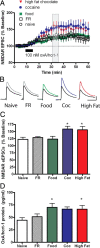
Orexin A/hypocretin-1 (oxA/hcrt-1) is known to be a modulator of dopamine-dependent neuronal activity and behaviors. However, the role of this system in driving motivated behaviors remains poorly understood. Here, we show that orexin/hypocretin receptor-1 (ox/hcrt-1R) signaling is important for motivation for highly salient, positive reinforcement. Blockade of ox/hcrt-1R selectively reduced work to self-administer cocaine or high fat food pellets. Moreover, oxA/hcrt-1 strengthened presynaptic glutamatergic inputs to the ventral tegmental area (VTA) only in cocaine or high fat self-administering rats. Finally, oxA/hcrt-1-mediated excitatory synaptic transmission onto VTA neurons was not potentiated following an arousing, aversive stimulus, suggesting that oxA/hcrt-1-mediated glutamatergic synaptic transmission was potentiated selectively with highly salient positive reinforcers. These experiments provide evidence for a selective role of oxA/hcrt-1 signaling in motivation for highly salient reinforcers and may represent a unique opportunity to design novel therapies that selectively reduce excessive drive to consume positive reinforcers of high salience.
Figures




References
-
- Akbari E, Naghdi N, Motamedi F. The selective orexin 1 receptor antagonist SB-334867-A impairs acquisition and consolidation but not retrieval of spatial memory in Morris water maze. Peptides. 2007;28:650–656. - PubMed
-
- Akbari E, Motamedi F, Naghdi N, Noorbakhshnia M. The effect of antagonization of orexin 1 receptors in CA1 and dentate gyrus regions on memory processing in passive avoidance task. Behav Brain Res. 2008;187:172–177. - PubMed
-
- Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J Comp Neurol. 2007;503:668–684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
