Abeta immunotherapy: intracerebral sequestration of Abeta by an anti-Abeta monoclonal antibody 266 with high affinity to soluble Abeta
- PMID: 19741145
- PMCID: PMC6665926
- DOI: 10.1523/JNEUROSCI.2021-09.2009
Abeta immunotherapy: intracerebral sequestration of Abeta by an anti-Abeta monoclonal antibody 266 with high affinity to soluble Abeta
Abstract
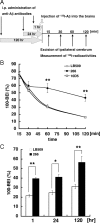
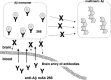
Amyloid beta (Abeta) immunotherapy is emerging as a promising disease-modifying therapy for Alzheimer's disease, although the precise mechanisms whereby anti-Abeta antibodies act against amyloid deposition and cognitive deficits remain elusive. To test the "peripheral sink" theory, which postulates that the effects of anti-Abeta antibodies in the systemic circulation are to promote the Abeta efflux from brain to blood, we studied the clearance of (125)I-Abeta(1-40) microinjected into mouse brains after intraperitoneal administration of an anti-Abeta monoclonal antibody 266. (125)I-Abeta(1-40) was rapidly eliminated from brains with a half-life of approximately 30 min in control mice, whereas 266 significantly retarded the elimination of Abeta, presumably due to formation of Abeta-antibody complex in brains. Administration of 266 to APP transgenic mice increased the levels of monomer Abeta species in an antibody-bound form, without affecting that of total Abeta. We propose a novel mechanism of Abeta immunotherapy by the class of anti-Abeta antibodies that preferentially bind soluble Abeta, i.e., intracerebral, rather than peripheral, sequestration of soluble, monomer form of Abeta, thereby preventing the accumulation of multimeric toxic Abeta species in brains.
Figures


References
-
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. - PubMed
-
- DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-β efflux: a measure of brain amyloid burden in a mouse model of Alzheimer's disease. Science. 2002a;295:2264–2267. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
