The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans
- PMID: 19741298
- PMCID: PMC2752076
- DOI: 10.1172/JCI39065
The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans
Abstract
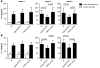
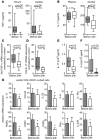
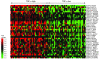
Cytokines orchestrate the tumor-promoting interplay between malignant cells and the immune system. In many experimental and human cancers, the cytokine TNF-alpha is an important component of this interplay, but its effects are pleiotropic and therefore remain to be completely defined. Using a mouse model of ovarian cancer in which either TNF receptor 1 (TNFR1) signaling was manipulated in different leukocyte populations or TNF-alpha was neutralized by antibody treatment, we found that this inflammatory cytokine maintained TNFR1-dependent IL-17 production by CD4+ cells and that this led to myeloid cell recruitment into the tumor microenvironment and enhanced tumor growth. Consistent with this, in patients with advanced cancer, treatment with the TNF-alpha-specific antibody infliximab substantially reduced plasma IL-17 levels. Furthermore, expression of IL-1R and IL-23R was downregulated in CD4+CD25- cells isolated from ascites of ovarian cancer patients treated with infliximab. We have also shown that genes ascribed to the Th17 pathway map closely with the TNF-alpha signaling pathway in ovarian cancer biopsy samples, showing particularly high levels of expression of genes encoding IL-23, components of the NF-kappaB system, TGF-beta1, and proteins involved in neutrophil activation. We conclude that chronic production of TNF-alpha in the tumor microenvironment increases myeloid cell recruitment in an IL-17-dependent manner that contributes to the tumor-promoting action of this proinflammatory cytokine.
Figures




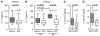
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

