Implications of chimaeric non-co-linear transcripts
- PMID: 19741701
- PMCID: PMC4020519
- DOI: 10.1038/nature08452
Implications of chimaeric non-co-linear transcripts
Abstract
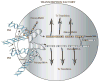
Deep sequencing of 'transcriptomes'--the collection of all RNA transcripts produced at a given time--from worms to humans reveals that some transcripts are composed of sequence segments that are not co-linear, with pieces of sequence coming from distant regions of DNA, even different chromosomes. Some of these 'chimaeric' transcripts are formed by genetic rearrangements, but others arise during post-transcriptional events. The 'trans-splicing' process in lower eukaryotes is well understood, but events in higher eukaryotes are not. The existence of such chimaeric RNAs has far-reaching implications for the potential information content of genomes and the way it is arranged.
Figures



References
-
- Jones PA, Archer TK, Baylin SB, Beck S, Berger S, Bernstein BE, Carpten JD, Clark SJ, Costello JF, Doerg RE, Esteller ME, Feinberg AP, Gingeras TR, Greally JM, Henikoff S, Herman JG, Jackson-Grusby L, Jenuwein T, Jirtle RL, Kim Y-J, Laird PW, Lim B, Martienssen R, Polyak K, Stunnenberg H, Tlsty TD, Tycko B, Ushijima T, Zhu J, Pirrotta V, Allis CD, Elgin S, Jones PA, Rine J, Wu C. Moving AHEAD with an international human epigenome project. Nature. 2008;454:711–715. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

