Repression of RNA polymerase II elongation in vivo is critically dependent on the C-terminus of Spt5
- PMID: 19742326
- PMCID: PMC2735033
- DOI: 10.1371/journal.pone.0006918
Repression of RNA polymerase II elongation in vivo is critically dependent on the C-terminus of Spt5
Abstract
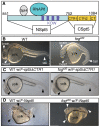
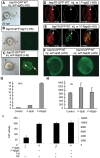
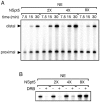
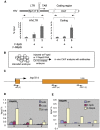
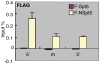
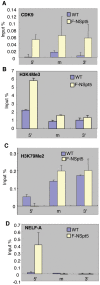
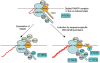
The stalling of RNA polymerase II (RNAPII) at the promoters of many genes, including developmental regulators, stress-responsive genes, and HIVLTR, suggests transcription elongation as a critical regulatory step in addition to initiation. Spt5, the large subunit of the DRB sensitivity-inducing factor (DSIF), represses or activates RNAPII elongation in vitro. How RNAPII elongation is repressed in vivo is not well understood. Here we report that CTR1 and CTR2CT, the two repeat-containing regions constituting the C-terminus of Spt5, play a redundant role in repressing RNAPII elongation in vivo. First, mis-expression of Spt5 lacking CTR1 or CTR2CT is inconsequential, but mis-expression of Spt5 lacking the entire C-terminus (termed NSpt5) dominantly impairs embryogenesis in zebrafish. Second, NSpt5 de-represses the transcription of hsp70-4 in zebrafish embryos and HIVLTR in cultured human cells, which are repressed at the RNAPII elongation step under non-inducible conditions. Third, NSpt5 directly associates with hsp70-4 chromatin in vivo and increases the occupancy of RNAPII, positive transcription elongation factor b (P-TEFb), histone H3 Lys 4 trimethylation (H3K4Me3), and surprisingly, the negative elongation factor A (NELF-A) at the locus, indicating a direct action of NSpt5 on the elongation repressed locus. Together, these results reveal a dominant activity of NSpt5 to de-repress RNAPII elongation, and suggest that the C-terminus of Spt5 is critical for repressing RNAPII elongation in vivo.
Conflict of interest statement
Figures
References
-
- Kadonaga JT. Regulation of RNA polymerase II transcription by sequence-specific DNA binding factors. Cell. 2004;116:247–257. - PubMed
-
- Saunders A, Core LJ, Lis JT. Breaking barriers to transcription elongation. Nat Rev Mol Cell Biol. 2006;7:557–567. - PubMed
-
- Kao SY, Calman AF, Luciw PA, Peterlin BM. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987;330:489–493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

