Pilot study of postexposure prophylaxis for hepatitis C virus in healthcare workers
- PMID: 19743901
- PMCID: PMC4331129
- DOI: 10.1086/605718
Pilot study of postexposure prophylaxis for hepatitis C virus in healthcare workers
Abstract
Background and objective: Hepatitis C virus (HCV) transmission occurs in 0.2%-10% of people after accidental needlestick exposures. However, postexposure prophylaxis is not currently recommended. We sought to determine the safety, tolerability, and acceptance of postexposure prophylaxis with peginterferon alfa-2b in healthcare workers (HCWs) exposed to blood from HCV-infected patients.
Design: Open-label pilot trial of peginterferon alfa-2b for HCV postexposure prophylaxis.
Setting: Two academic tertiary-referral centers.
Methods: HCWs exposed to blood from HCV-infected patients were informed of the availability of postexposure prophylaxis. Persons who elected postexposure prophylaxis were given weekly doses of peginterferon alfa-2b for 4 weeks.
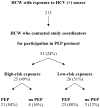
Results: Among 2,702 HCWs identified with potential exposures to bloodborne pathogens, 213 (7.9%) were exposed to an HCV antibody-positive source. Of 51 HCWs who enrolled in the study, 44 (86%) elected to undergo postexposure prophylaxis (treated group). Seven subjects elected not to undergo postexposure prophylaxis (untreated group). No cases of HCV transmission were observed in either the treated or untreated group, and no cases occurred in the remaining 162 HCWs who did not enroll in this study. No serious adverse events related to a peginterferon alfa-2b regimen were recorded, but minor adverse events were frequent.
Conclusion: In this pilot study, there was a lower than expected frequency of HCV transmission after accidental occupational exposure. Although peginterferon alfa-2b was safe, because of the lack of HCV transmission in either the treated or untreated groups there is little evidence to support routine postexposure prophylaxis against HCV in HCWs.
Conflict of interest statement
All remaining authors have no conflicts of interest to report.
Figures
References
-
- Chung H, Kudo M, Kumada T, et al. Risk of HCV transmission after needlestick injury, and the efficacy of short-duration interferon administration to prevent HCV transmission to medical personnel. J Gastroenterol. 2003;38(9):877–9. - PubMed
-
- Jagger J, Puro V, De Carli G. Occupational transmission of hepatitis C virus. Jama. 2002;288(12):1469. author reply 1469–71. - PubMed
-
- Yen T, Keeffe EB, Ahmed A. The epidemiology of hepatitis C virus infection. J Clin Gastroenterol. 2003;36(1):47–53. - PubMed
-
- Takagi H, Uehara M, Kakizaki S, et al. Accidental transmission of HCV and treatment with interferon. J Gastroenterol Hepatol. 1998;13(3):238–43. - PubMed
-
- Mitsui T, Iwano K, Masuko K, et al. Hepatitis C virus infection in medical personnel after needlestick accident. Hepatology. 1992;16(5):1109–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical