Dendritic cell therapy of high-grade gliomas
- PMID: 19744041
- PMCID: PMC8094646
- DOI: 10.1111/j.1750-3639.2009.00316.x
Dendritic cell therapy of high-grade gliomas
Abstract
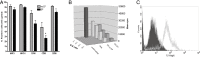
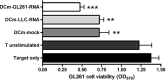
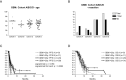
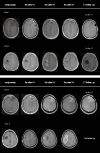
The prognosis of patients with malignant glioma is poor in spite of multimodal treatment approaches consisting of neurosurgery, radiochemotherapy and maintenance chemotherapy. Among innovative treatment strategies like targeted therapy, antiangiogenesis and gene therapy approaches, immunotherapy emerges as a meaningful and feasible treatment approach for inducing long-term survival in at least a subpopulation of these patients. Setting up immunotherapy for an inherent immunosuppressive tumor located in an immune-privileged environment requires integration of a lot of scientific input and knowledge of both tumor immunology and neuro-oncology. The field of immunotherapy is moving into the direction of active specific immunotherapy using autologous dendritic cells (DCs) as vehicle for immunization. In the translational research program of the authors, the whole cascade from bench to bed to bench of active specific immunotherapy for malignant glioma is covered, including proof of principle experiments to demonstrate immunogenicity of patient-derived mature DCs loaded with autologous tumor lysate, preclinical in vivo experiments in a murine orthotopic glioma model, early phase I/II clinical trials for relapsing patients, a phase II trial for patients with newly diagnosed glioblastoma (GBM) for whom immunotherapy is integrated in the current multimodal treatment, and laboratory analyses of patient samples. The strategies and results of this program are discussed in the light of the internationally available scientific literature in this fast-moving field of basic science and translational clinical research.
Figures

References
-
- Akasaki Y, Black KL, Yu JS (2005) Dendritic cell‐based immunotherapy for malignant gliomas. Expert Rev Neurother 5:497–508. - PubMed
-
- Akasaki Y, Black KL, Yu JS (2005) T cell immunity in patients with malignant glioma: recent progress in dendritic cell‐based immunotherapeutic approaches. Front Biosci 10:2908–2921. - PubMed
-
- Akasaki Y, Liu G, Chung NH, Ehtesham M, Black KL, Yu JS (2004) Induction of a CD4+ T regulatory type 1 response by cyclooxygenase‐2‐overexpressing glioma. J Immunol 173:4352–4359. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

