EGFRvIII-targeted vaccination therapy of malignant glioma
- PMID: 19744042
- PMCID: PMC2846812
- DOI: 10.1111/j.1750-3639.2009.00318.x
EGFRvIII-targeted vaccination therapy of malignant glioma
Abstract
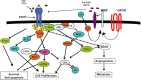
Given the highly infiltrative growth pattern of malignant glioma and the lack of specificity associated with currently available treatment regimens, alternative strategies designed to eradicate cancer cells while limiting collateral toxicity in normal tissues remain a high priority. To this end, the development of specific immunotherapies against targeted neoplastic cells represents a promising approach. The epidermal growth factor receptor class III variant (EGFRvIII), a constitutively activated mutant of the wild-type tyrosine kinase, is present in a substantial proportion of malignant gliomas and other human cancers, yet completely absent from normal tissues. This receptor variant consists of an in-frame deletion, the translation of which produces an extracellular junction with a novel glycine residue, flanked by amino acid sequences that are not typically adjacent in the normal protein. In this review, both preclinical and early clinical development of a peptide vaccine directed against this portion of the EGFRvIII antigenic domain are recapitulated. Following vaccination, our group has demonstrated potent, redirected cellular and humoral immunity against cancer cells expressing the mutant receptor without significant toxicity. Additionally, the corresponding therapeutic outcomes observed in these studies lend credence to the potential role of peptide-based vaccination strategies among emerging antitumor immunotherapies in patients with malignant glioma.
Figures


References
-
- Aloisi F, Ria F, Adorini L (2000) Regulation of T‐cell responses by CNS antigen‐presenting cells: different roles for microglia and astrocytes. Immunol Today 21:141–147. - PubMed
-
- Antonyak MA, Moscatello DK, Wong AJ (1998) Constitutive activation of c‐Jun N‐terminal kinase by a mutant epidermal growth factor receptor. J Biol Chem 273:2817–2822. - PubMed
-
- Bacskai BJ, Kajdasz ST, Christie RH, Carter C, Games D, Seubert P et al (2001) Imaging of amyloid‐beta deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat Med 7:369–372. - PubMed
-
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H et al (2000) Peripherally administered antibodies against amyloid beta‐peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 6:916–919. - PubMed
-
- Batra SK, Castelino‐Prabhu S, Wikstrand CJ, Zhu X, Humphrey PA, Friedman HS, Bigner DD (1995) Epidermal growth factor ligand‐independent, unregulated, cell‐transforming potential of a naturally occurring human mutant EGFRvIII gene. Cell Growth Differ 6:1251–1259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

