Energetic performance is improved by specific activation of K+ fluxes through K(Ca) channels in heart mitochondria
- PMID: 19744465
- PMCID: PMC2787855
- DOI: 10.1016/j.bbabio.2009.08.002
Energetic performance is improved by specific activation of K+ fluxes through K(Ca) channels in heart mitochondria
Abstract
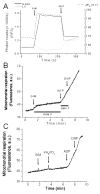
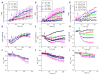
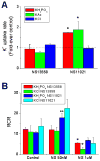
Mitochondrial volume regulation depends on K+ movement across the inner membrane and a mitochondrial Ca2+-dependent K+ channel (mitoK(Ca)) reportedly contributes to mitochondrial K+ uniporter activity. Here we utilize a novel K(Ca) channel activator, NS11021, to examine the role of mitoK(Ca) in regulating mitochondrial function by measuring K+ flux, membrane potential (DeltaPsi(m)), light scattering, and respiration in guinea pig heart mitochondria. K+ uptake and the influence of anions were assessed in mitochondria loaded with the K+ sensor PBFI by adding either the chloride (KCl), acetate (KAc), or phosphate (KH2PO4) salts of K+ to energized mitochondria in a sucrose-based medium. K+ fluxes saturated at approximately 10 mM for each salt, attaining maximal rates of 172+/-17, 54+/-2.4, and 33+/-3.8 nmol K+/min/mg in KCl, KAc, or KH2PO4, respectively. NS11021 (50 nM) increased the maximal K+ uptake rate by 2.5-fold in the presence of KH2PO4 or KAc and increased mitochondrial volume, with little effect on DeltaPsi(m). In KCl, NS11021 increased K+ uptake by only 30% and did not increase volume. The effects of NS11021 on K+ uptake were inhibited by the K(Ca) toxins charybdotoxin (200 nM) or paxilline (1 microM). Fifty nanomolar of NS11021 increased the mitochondrial respiratory control ratio (RCR) in KH2PO4, but not in KCl; however, above 1 microM, NS11021 decreased RCR and depolarized DeltaPsi(m). A control compound lacking K(Ca) activator properties did not increase K+ uptake or volume but had similar nonspecific (toxin-insensitive) effects at high concentrations. The results indicate that activating K+ flux through mitoK(Ca) mediates a beneficial effect on energetics that depends on mitochondrial swelling with maintained DeltaPsi(m).
Figures





References
-
- Garlid KD, Dos Santos P, Xie ZJ, Costa AD, Paucek P. Mitochondrial potassium transport: the role of the mitochondrial ATP-sensitive K(+) channel in cardiac function and cardioprotection. Biochimica et biophysica acta. 2003;1606:1–21. - PubMed
-
- Bednarczyk P, Barker GD, Halestrap AP. Determination of the rate of K(+) movement through potassium channels in isolated rat heart and liver mitochondria. Biochimica et biophysica acta. 2008;1777:540–548. - PubMed
-
- Kaasik A, Safiulina D, Zharkovsky A, Veksler V. Regulation of mitochondrial matrix volume. Am J Physiol Cell Physiol. 2007;292:C157–163. - PubMed
-
- Brierley GP. The uptake and extrusion of monovalent cations by isolated heart mitochondria. Mol Cell Biochem. 1976;10:41–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

