Intersection based motion correction of multislice MRI for 3-D in utero fetal brain image formation
- PMID: 19744911
- PMCID: PMC3328314
- DOI: 10.1109/TMI.2009.2030679
Intersection based motion correction of multislice MRI for 3-D in utero fetal brain image formation
Abstract
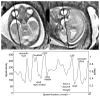
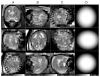
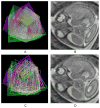
In recent years, postprocessing of fast multislice magnetic resonance imaging (MRI) to correct fetal motion has provided the first true 3-D MR images of the developing human brain in utero. Early approaches have used reconstruction based algorithms, employing a two-step iterative process, where slices from the acquired data are realigned to an approximate 3-D reconstruction of the fetal brain, which is then refined further using the improved slice alignment. This two step slice-to-volume process, although powerful, is computationally expensive in needing a 3-D reconstruction, and is limited in its ability to recover subvoxel alignment. Here, we describe an alternative approach which we term slice intersection motion correction (SIMC), that seeks to directly co-align multiple slice stacks by considering the matching structure along all intersecting slice pairs in all orthogonally planned slices that are acquired in clinical imaging studies. A collective update scheme for all slices is then derived, to simultaneously drive slices into a consistent match along their lines of intersection. We then describe a 3-D reconstruction algorithm that, using the final motion corrected slice locations, suppresses through-plane partial volume effects to provide a single high isotropic resolution 3-D image. The method is tested on simulated data with known motions and is applied to retrospectively reconstruct 3-D images from a range of clinically acquired imaging studies. The quantitative evaluation of the registration accuracy for the simulated data sets demonstrated a significant improvement over previous approaches. An initial application of the technique to studying clinical pathology is included, where the proposed method recovered up to 15 mm of translation and 30 degrees of rotation for individual slices, and produced full 3-D reconstructions containing clinically useful additional information not visible in the original 2-D slices.
Figures
















References
-
- Weinreb J, Lowe T, Cohen J, Kutler M. Human fetal anatomy: MR imaging. Radiology. 1985;157:715–720. - PubMed
-
- Lowe T, Weinreb J, Santos-Ramos R, Cunningham F. Magnetic resonance imaging in human pregnancy. Obstet Gynecol. 1985;66:629–623. - PubMed
-
- Horvath L, Seeds J. Temporary arrest of fetal movement with pancuronium bromide to enable antenal magnetic resonance imaging of hologrosencephaly. Am J Perinatol. 1989;6:418–420. - PubMed
-
- Lenke R, Persutte W, Nemes J. Use of pancuronium bromide to inhibit fetal movement during magnetic resonance imaging. J Reprod Med. 1989;34:315–317. - PubMed
-
- Mansfield P, Stehling M, Ordidge R, Coxon R, Chapman B, Blamire A, Gibbs P, Johnson I, Symonds E, Worthington B, Coupland R. Echo planar imaging of the human fetus in utero at 0.5T. Br J Radiol. 1990;63:833–841. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

