The transmissibility and control of pandemic influenza A (H1N1) virus
- PMID: 19745114
- PMCID: PMC2880578
- DOI: 10.1126/science.1177373
The transmissibility and control of pandemic influenza A (H1N1) virus
Abstract
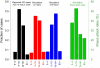
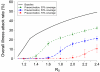
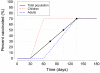
Pandemic influenza A (H1N1) 2009 (pandemic H1N1) is spreading throughout the planet. It has become the dominant strain in the Southern Hemisphere, where the influenza season has now ended. Here, on the basis of reported case clusters in the United States, we estimated the household secondary attack rate for pandemic H1N1 to be 27.3% [95% confidence interval (CI) from 12.2% to 50.5%]. From a school outbreak, we estimated that a typical schoolchild infects 2.4 (95% CI from 1.8 to 3.2) other children within the school. We estimated the basic reproductive number, R0, to range from 1.3 to 1.7 and the generation interval to range from 2.6 to 3.2 days. We used a simulation model to evaluate the effectiveness of vaccination strategies in the United States for fall 2009. If a vaccine were available soon enough, vaccination of children, followed by adults, reaching 70% overall coverage, in addition to high-risk and essential workforce groups, could mitigate a severe epidemic.
Figures








References
-
- World Health Organization Novel Influenza A (H1N1) - Update 59. [accessed July 28, 2009]. Jul 272009. http://www.who.int/csr/don/2009_07_27/en/index.html.
-
- Chan M. World now at start of 2009 influenza pandemic. statement to the press by WHO director-general. [accessed July 28, 2009]. Jun 112009. http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6....
-
- Centers for Disease Control and Prevention Swine Influenza A (H1N1) infection in two children–Southern California, March-April 2009. 2009. http://www.cdc.gov/mmwr/PDF/wk/mm5815.pdf. - PubMed
-
- Center for Diseases Control and Prevention Update: swine influenza A (H1N1) infections–California and Texas, April 2009. 2009. http://www.cdc.gov/mmwr/PDF/wk/mm5816.pdf. - PubMed
-
- Kansas Department of Health and Environment Swine influenza news conference. [accessed April 29, 2009]. Apr 292009. http://www.dhe.state.ks.us/SwineFlu/swineflunewsconf.wmv.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

