Losartan chemistry and its effects via AT1 mechanisms in the kidney
- PMID: 19747145
- PMCID: PMC2819278
- DOI: 10.2174/092986709789105000
Losartan chemistry and its effects via AT1 mechanisms in the kidney
Abstract
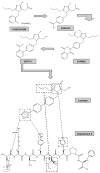
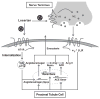
Besides the importance of the renin-angiotensin system (RAS) in the circulation and other organs, the local RAS in the kidney has attracted a great attention in research in last decades. The renal RAS plays an important role in the body fluid homeostasis and long-term cardiovascular regulation. All major components and key enzymes for the establishment of a local RAS as well as two important angiotensin II (Ang II) receptor subtypes, AT1 and AT2 receptors, have been confirmed in the kidney. In additional to renal contribution to the systemic RAS, the intrarenal RAS plays a critical role in the regulation of renal function as well as in the development of kidney disease. Notably, kidney AT1 receptors locating at different cells and compartments inside the kidney are important for normal renal physiological functions and abnormal pathophysiological processes. This mini-review focuses on: 1) the local renal RAS and its receptors, particularly the AT1 receptor and its mechanisms in physiological and pathophysiological processes; and 2) the chemistry of the selective AT1 receptor blocker, losartan, and the potential mechanisms for its actions in the renal RAS-mediated disease.
Figures
Similar articles
-
Intrarenal Mas and AT1 receptors play a role in mediating the excretory actions of renal interstitial angiotensin-(1-7) infusion in anaesthetized rats.Exp Physiol. 2017 Dec 1;102(12):1700-1715. doi: 10.1113/EP086513. Epub 2017 Nov 2. Exp Physiol. 2017. PMID: 28940861
-
Impact of early life AT1 blockade on adult cardiac morpho-functional changes and the renin-angiotensin system in a model of neonatal high oxygen-induced cardiomyopathy.Eur J Pharmacol. 2019 Oct 5;860:172585. doi: 10.1016/j.ejphar.2019.172585. Epub 2019 Jul 31. Eur J Pharmacol. 2019. PMID: 31376367
-
Modulation of renal glomerular angiotensin II receptors by ace inhibition and AT1 receptor antagonism.Regul Pept. 1997 Jan 29;68(2):111-7. doi: 10.1016/s0167-0115(96)02112-x. Regul Pept. 1997. PMID: 9110382
-
Cardiovascular effects of losartan and its relevant clinical application.Curr Med Chem. 2009;16(29):3841-57. doi: 10.2174/092986709789178046. Curr Med Chem. 2009. PMID: 19747137 Free PMC article. Review.
-
Mapping tissue angiotensin-converting enzyme and angiotensin AT1, AT2 and AT4 receptors.J Hypertens. 1998 Dec;16(12 Pt 2):2027-37. doi: 10.1097/00004872-199816121-00026. J Hypertens. 1998. PMID: 9886893 Review.
Cited by
-
Chronic kidney disease and aging: dissecting the p53/p21 pathway as a therapeutic target.Biogerontology. 2024 Dec 26;26(1):32. doi: 10.1007/s10522-024-10173-z. Biogerontology. 2024. PMID: 39725742 Review.
-
High-salt diets during pregnancy affected fetal and offspring renal renin-angiotensin system.J Endocrinol. 2013 Jun 1;218(1):61-73. doi: 10.1530/JOE-13-0139. Print 2013 Jul. J Endocrinol. 2013. PMID: 23620529 Free PMC article.
-
Effects of Xuesaitong on the Pharmacokinetics of Losartan: An In Vivo UPLC-MS/MS Study.Evid Based Complement Alternat Med. 2019 Aug 14;2019:8373476. doi: 10.1155/2019/8373476. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 31511782 Free PMC article.
-
Changed salt appetite and central angiotensin II-induced cellular activation in rat offspring following hypoxia during fetal stages.Peptides. 2010 Jun;31(6):1177-83. doi: 10.1016/j.peptides.2010.03.009. Epub 2010 Mar 20. Peptides. 2010. PMID: 20307607 Free PMC article.
-
Altered dipsogenic responses and expression of angiotensin receptors in the offspring exposed to prenatal high sucrose.Peptides. 2011 Jan;32(1):104-11. doi: 10.1016/j.peptides.2010.10.012. Epub 2010 Oct 19. Peptides. 2011. PMID: 20965221 Free PMC article.
References
-
- Ram CVS. Angiotensin Receptor Blockers: Current Status and Future Prospects. Am J Med. 2008;121(8):656–663. - PubMed
-
- Billet S, Aguilar F, Baudry C, Clauser E. Role of angiotensin II AT1 receptor activation in cardiovascular diseases. Kidney Int. 2008;74(11):1379–1384. - PubMed
-
- Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59(3):251–287. - PubMed
-
- Ruilope LM. Angiotensin receptor blockers: RAAS blockade and renoprotection. Curr Med Res Opin. 2008;24(5):1285–1293. - PubMed
-
- Wolf G. Novel aspects of the renin-angiotensin-aldosterone-system. Front Biosci. 2008;13:4993–5005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

