Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner
- PMID: 19747917
- PMCID: PMC2862622
- DOI: 10.1053/j.gastro.2009.08.066
Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner
Abstract
Background & aims: Patients with chronic hepatitis C virus (HCV) infection display great variability in disease activity and progression. Although virus-specific adaptive immune responses have been characterized extensively and found to be impaired in chronic hepatitis C, the role of innate immune responses in disease activity and progression of chronic hepatitis C is not well understood.
Methods: We studied 42 HCV-infected patients and 12 healthy uninfected controls.
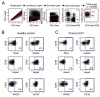
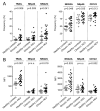
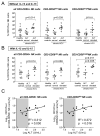
Results: We found an increased frequency of natural killer (NK) cells expressing tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), NKp44, NKG2C, and CD122 in chronic hepatitis C as compared with healthy controls (P < .05 for all markers) and stronger activation of NK cells in the liver than in the blood (P < .05). This NK cell phenotype was associated with polarization of NK cell function toward CD107a expression as a marker of degranulation, but with not increased interferon (IFN)-gamma production of CD56(dim) NK cells. The polarized NK cell phenotype correlated with alanine aminotransferase levels (r(2) = 0.312, P = .03). To investigate whether in vivo exposure of NK cells to HCV-induced type I IFN was causing this NK cell phenotype, peripheral blood mononuclear cells from 10 healthy controls and 8 HCV-infected patients were stimulated in the presence of IFN-alfa, which resulted in increased NK cell expression of TRAIL and CD107a (P < .001), but not IFN-gamma.
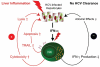
Conclusions: Collectively, these results describe a polarized NK cell phenotype induced by chronic exposure to HCV-induced IFN-alfa. This phenotype may contribute to liver injury through TRAIL expression and cytotoxicity, whereas the lacking increase in IFN-gamma production may facilitate the inability to clear HCV.
Copyright 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–29. - PubMed
-
- Chang KM, Thimme R, Melpolder JJ, Oldach D, Pemberton J, Moorhead-Loudis J, McHutchison JG, Alter HJ, Chisari FV. Differential CD4 and CD8 T-cell responsiveness in hepatitis C virus infection. Hepatology. 2001;33:267–276. - PubMed
-
- Lauer GM, Barnes E, Lucas M, Timm J, Ouchi K, Kim AY, Day CL, Robbins GK, Casson DR, Reiser M, Dusheiko G, Allen TM, Chung RT, Walker BD, Klenerman P. High resolution analysis of cellular immune responses in resolved and persistent hepatitis C virus infection. Gastroenterology. 2004;127:924–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

