The plasmodium receptor for activated C kinase protein inhibits Ca(2+) signaling in mammalian cells
- PMID: 19748487
- PMCID: PMC2762016
- DOI: 10.1016/j.bbrc.2009.09.025
The plasmodium receptor for activated C kinase protein inhibits Ca(2+) signaling in mammalian cells
Abstract
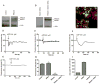
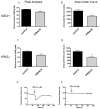
Plasmodium falciparum, the most lethal malarial parasite, expresses an ortholog for the protein kinase C (PKC) activator RACK1. However, PKC has not been identified in this parasite, and the mammalian RACK1 can interact with the inositol 1,4,5-trisphosphate receptor (InsP3R). Therefore we investigated whether the Plasmodium ortholog PfRACK also can affect InsP3R-mediated Ca(2+) signaling in mammalian cells. GFP-tagged PfRACK and endogenous RACK1 were expressed in a similar distribution within cells. PfRACK inhibited agonist-induced Ca(2+) signals in cells expressing each isoform of the InsP3R, and this effect persisted when expression of endogenous RACK1 was reduced by siRNA. PfRACK also inhibited Ca(2+) signals induced by photorelease of caged InsP3. These findings provide evidence that PfRACK directly inhibits InsP3-mediated Ca(2+) signaling in mammalian cells. Interference with host cell signaling pathways to subvert the host intracellular milieu may be an important mechanism for parasite survival.
Figures


References
-
- Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell. 2004;117:503–514. - PubMed
-
- Vaid A, Sharma P. PfPKB, a protein kinase B-like enzyme from Plasmodium falciparum II. Identification of calcium/calmodulin as its upstream activator and dissection of a novel signaling pathway. Journal of Biological Chemistry. 2006;281:27126–27133. - PubMed
-
- Vaid A, Sharma P. PfPKB, a protein kinase B-like enzyme from Plasmodium falciparum II. Identification of calcium/calmodulin as its upstream activator and dissection of a novel signaling pathway. Journal of Biological Chemistry. 2006;281:27126–27133. - PubMed
-
- Hotta CT, Gazarini ML, Beraldo FH, Varotti FP, Lopes C, Markus RP, Pozzan T, Garcia CRS. Calcium-dependent modulation by melatonin of the circadian rhythm in malarial parasites. Nature Cell Biology. 2000;2:466–468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

