Crowded cell-like environment accelerates the nucleation step of amyloidogenic protein misfolding
- PMID: 19748895
- PMCID: PMC2781570
- DOI: 10.1074/jbc.M109.002832
Crowded cell-like environment accelerates the nucleation step of amyloidogenic protein misfolding
Abstract
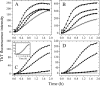
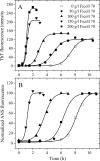
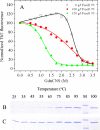
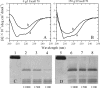
To understand the role of a crowded physiological environment in the pathogenesis of neurodegenerative diseases, we report the following. 1) The formation of fibrous aggregates of the human Tau fragment Tau-(244-441), when hyperphosphorylated by glycogen synthase kinase-3beta, is dramatically facilitated by the addition of crowding agents. 2) Fibril formation of nonphosphorylated Tau-(244-441) is only promoted moderately by macromolecular crowding. 3) Macromolecular crowding dramatically accelerates amyloid formation by human prion protein. A sigmoidal equation has been used to fit these kinetic data, including published data of human alpha-synuclein, yielding lag times and apparent rate constants for the growth of fibrils for these amyloidogenic proteins. These biochemical data indicate that crowded cell-like environments significantly accelerate the nucleation step of fibril formation of human Tau fragment/human prion protein/human alpha-synuclein (a significant decrease in the lag time). These results can in principle be predicted based on some known data concerning protein concentration effects on fibril formation both in vitro and in vivo. Furthermore, macromolecular crowding causes human prion protein to form short fibrils and nonfibrillar particles with lower conformational stability and higher protease resistance activity, compared with those formed in dilute solutions. Our data demonstrate that a crowded physiological environment could play an important role in the pathogenesis of neurodegenerative diseases by accelerating amyloidogenic protein misfolding and inducing human prion fibril fragmentation, which is considered to be an essential step in prion replication.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

