Spatiotemporal control of cell signalling using a light-switchable protein interaction
- PMID: 19749742
- PMCID: PMC2989900
- DOI: 10.1038/nature08446
Spatiotemporal control of cell signalling using a light-switchable protein interaction
Abstract
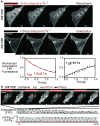
Genetically encodable optical reporters, such as green fluorescent protein, have revolutionized the observation and measurement of cellular states. However, the inverse challenge of using light to control precisely cellular behaviour has only recently begun to be addressed; semi-synthetic chromophore-tethered receptors and naturally occurring channel rhodopsins have been used to perturb directly neuronal networks. The difficulty of engineering light-sensitive proteins remains a significant impediment to the optical control of most cell-biological processes. Here we demonstrate the use of a new genetically encoded light-control system based on an optimized, reversible protein-protein interaction from the phytochrome signalling network of Arabidopsis thaliana. Because protein-protein interactions are one of the most general currencies of cellular information, this system can, in principle, be generically used to control diverse functions. Here we show that this system can be used to translocate target proteins precisely and reversibly to the membrane with micrometre spatial resolution and at the second timescale. We show that light-gated translocation of the upstream activators of Rho-family GTPases, which control the actin cytoskeleton, can be used to precisely reshape and direct the cell morphology of mammalian cells. The light-gated protein-protein interaction that has been optimized here should be useful for the design of diverse light-programmable reagents, potentially enabling a new generation of perturbative, quantitative experiments in cell biology.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

