Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging
- PMID: 19749772
- PMCID: PMC2759417
- DOI: 10.1038/nm.1971
Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging
Abstract
Intravital multiphoton microscopy has provided powerful mechanistic insights into health and disease and has become a common instrument in the modern biological laboratory. The requisite high numerical aperture and exogenous contrast agents that enable multiphoton microscopy, however, limit the ability to investigate substantial tissue volumes or to probe dynamic changes repeatedly over prolonged periods. Here we introduce optical frequency domain imaging (OFDI) as an intravital microscopy that circumvents the technical limitations of multiphoton microscopy and, as a result, provides unprecedented access to previously unexplored, crucial aspects of tissue biology. Using unique OFDI-based approaches and entirely intrinsic mechanisms of contrast, we present rapid and repeated measurements of tumor angiogenesis, lymphangiogenesis, tissue viability and both vascular and cellular responses to therapy, thereby demonstrating the potential of OFDI to facilitate the exploration of physiological and pathological processes and the evaluation of treatment strategies.
Figures



 ). (d) HSTS26T tumor (blue asterisk, *) associated lymphatics exhibiting hyperplasia. (e) Cross-sectional presentations of a lymphatic vessel showing cellular masses (yellow arrowhead,
). (d) HSTS26T tumor (blue asterisk, *) associated lymphatics exhibiting hyperplasia. (e) Cross-sectional presentations of a lymphatic vessel showing cellular masses (yellow arrowhead,  ) located near the tumor in d. Scale bars, 500 μm.
) located near the tumor in d. Scale bars, 500 μm.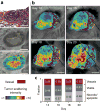

References
-
- Masters BR, So PT. Handbook of Biomedical Nonlinear Optical Microscopy. Oxford University Press; New York: 2008.
-
- Brown EB, et al. In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nat Med. 2001;7:864–868. - PubMed
-
- Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005:58–62. - PubMed
Publication types
MeSH terms
Grants and funding
- T32 CA073479/CA/NCI NIH HHS/United States
- R01-CA085140/CA/NCI NIH HHS/United States
- R00 CA137167/CA/NCI NIH HHS/United States
- R01 CA085140/CA/NCI NIH HHS/United States
- R01-CA115767/CA/NCI NIH HHS/United States
- K25-CA127465/CA/NCI NIH HHS/United States
- P01-CA080124/CA/NCI NIH HHS/United States
- P01 CA080124/CA/NCI NIH HHS/United States
- R01 CA115767/CA/NCI NIH HHS/United States
- R01 CA126642/CA/NCI NIH HHS/United States
- R33-CA125560/CA/NCI NIH HHS/United States
- K25 CA127465/CA/NCI NIH HHS/United States
- R24 CA085140/CA/NCI NIH HHS/United States
- K99-CA137167/CA/NCI NIH HHS/United States
- R33 CA125560/CA/NCI NIH HHS/United States
- R01-CA096915/CA/NCI NIH HHS/United States
- R01 CA096915/CA/NCI NIH HHS/United States
- K99 CA137167/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

