Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression
- PMID: 19750228
- PMCID: PMC2736622
- DOI: 10.1371/journal.pone.0006951
Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression
Abstract
Background: Niemann-Pick type C (NPC) disease is a fatal neurodegenerative disorder caused most commonly by a defect in the NPC1 protein and characterized by widespread intracellular accumulation of unesterified cholesterol and glycosphingolipids (GSLs). While current treatment therapies are limited, a few drugs tested in Npc1(-/-) mice have shown partial benefit. During a combination treatment trial using two such compounds, N-butyldeoxynojirimycin (NB-DNJ) and allopregnanolone, we noted increased lifespan for Npc1(-/-) mice receiving only 2-hydroxypropyl-beta-cyclodextrin (CD), the vehicle for allopregnanolone. This finding suggested that administration of CD alone, but with greater frequency, might provide additional benefit.
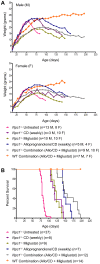
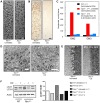
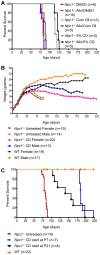
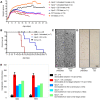
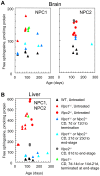
Methodology/principal findings: Administration of CD to Npc1(-/-) mice beginning at either P7 or P21 and continuing every other day delayed clinical onset, reduced intraneuronal cholesterol and GSL storage as well as free sphingosine accumulation, reduced markers of neurodegeneration, and led to longer survival than any previous treatment regime. We reasoned that other lysosomal diseases characterized by cholesterol and GSL accumulation, including NPC disease due to NPC2 deficiency, GM1 gangliosidosis and mucopolysaccharidosis (MPS) type IIIA, might likewise benefit from CD treatment. Treated Npc2(-/-) mice showed benefits similar to NPC1 disease, however, mice with GM1 gangliosidosis or MPS IIIA failed to show reduction in storage.
Conclusions/significance: Treatment with CD delayed clinical disease onset, reduced intraneuronal storage and secondary markers of neurodegeneration, and significantly increased lifespan of both Npc1(-/-) and Npc2(-/-) mice. In contrast, CD failed to ameliorate cholesterol or glycosphingolipid storage in GM1 gangliosidosis and MPS IIIA disease. Understanding the mechanism(s) by which CD leads to reduced neuronal storage may provide important new opportunities for treatment of NPC and related neurodegenerative diseases characterized by cholesterol dyshomeostasis.
Conflict of interest statement
Figures



References
-
- Patterson MC, Vanier MT, Suzuki K, Morris JA, Carstea E, et al. Niemann-Pick disease type C: a lipid trafficking disorder. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. New York: McGraw-Hill Medical Publishing Division; 2001. pp. 3611–3633. The Metabolic & Molecular Bases of Inherited Disease, vol. 3, 8th edition.
-
- Walkley SU, Suzuki K. Consequences of NPC1 and NPC2 loss of function in mammalian neurons. Biochim Biophys Acta. 2004;1685:48–62. - PubMed
-
- Sarna JR, Larouche M, Marzban H, Sillitoe RV, Rancourt DE, et al. Patterned Purkinje cell degeneration in mouse models of Niemann-Pick type C disease. J Comp Neurol. 2003;456:279–291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

