Evidence that cathelicidin peptide LL-37 may act as a functional ligand for CXCR2 on human neutrophils
- PMID: 19750480
- PMCID: PMC3076219
- DOI: 10.1002/eji.200939496
Evidence that cathelicidin peptide LL-37 may act as a functional ligand for CXCR2 on human neutrophils
Abstract
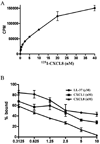
LL-37, derived from human cathelicidin, stimulates immune responses in neutrophils. Although FPR2 and P2X7 were proposed as LL-37 receptors, we have shown that among 21 neutrophil receptors only CXCR2 was down-regulated by LL-37. LL-37 functions similarly to CXCR2-specific chemokines CXCL1 and CXCL7 in terms of receptor down-regulation and intracellular calcium mobilization on freshly isolated neutrophils. Neutrophils pretreated with CXCL8, a chemokine that binds both CXCR1/2, completely blocked the calcium mobilization in response to LL-37, while LL-37 also partially inhibited (125)I-CXCL8 binding to neutrophils. SB225002, a selective CXCR2 antagonist, blocked LL-37-induced calcium mobilization and migration of neutrophils. LL-37 stimulates calcium mobilization in CXCR2-transfected HEK293 cells, CXCR2(+) THP-1 cells and monocytes, but not in CXCR1-transfected HEK293 cells. WKYMVm peptide (ligand for FPR2) does not block LL-37-stimulated calcium flux in either THP-1 (FPR2(-)) or monocytes (FPR2(high)), further confirming the specificity of LL-37 for CXCR2 and not FPR2. Among all ligands tested (ATP, BzATP, WKYMVm, CXCL1, and LL-37), only LL-37 stimulated migration of monocytes (CXCR2(+) and FPR2(+)) and migration was inhibited by the CXCR2 inhibitor SB225002. Moreover, CXCR2 but not CXCR1 was internalized in LL-37-treated neutrophils. Thus, our data provide evidence that LL-37 may act as a functional ligand for CXCR2 on human neutrophils.
Conflict of interest statement
Figures







References
-
- Sorensen O, Arnljots K, Cowland JB, Bainton DF, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–2803. - PubMed
-
- Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, Kiessling R, Jornvall H, Wigzell H, Gudmundsson GH. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. - PubMed
-
- Sorensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS, Hiemstra PS, Borregaard N. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–3959. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

