Lymphatic development
- PMID: 19750516
- PMCID: PMC2755610
- DOI: 10.1002/bdrc.20155
Lymphatic development
Abstract
The lymphatic system is essential for fluid homeostasis, immune responses, and fat absorption, and is involved in many pathological processes, including tumor metastasis and lymphedema. Despite its importance, progress in understanding the origins and early development of this system has been hampered by lack of defining molecular markers and difficulties in observing lymphatic cells in vivo and performing genetic and experimental manipulation of the lymphatic system. Recent identification of new molecular markers, new genes with important functional roles in lymphatic development, and new experimental models for studying lymphangiogenesis has begun to yield important insights into the emergence and assembly of this important tissue. This review focuses on the mechanisms regulating development of the lymphatic vasculature during embryogenesis.
Figures


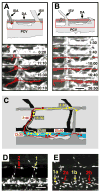
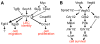
References
-
- Abtahian F, Guerriero A, Sebzda E, Lu MM, Zhou R, Mocsai A, Myers EE, Huang B, Jackson DG, Ferrari VA, Tybulewicz V, Lowell CA, Lepore JJ, Koretzky GA, Kahn ML. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science (New York, NY. 2003;299(5604):247–251. - PMC - PubMed
-
- Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proceedings of the National Academy of Sciences of the United States of America. 1998;95(2):548–553. - PMC - PubMed
-
- Aselli G. De Lacteibus sive Lacteis Venis, Quarto Vasorum Mesarai corum Genere novo invento. Milan: Mediolani; 1627.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

