Viruses and autophagy
- PMID: 19750559
- PMCID: PMC2852112
- DOI: 10.1002/rmv.630
Viruses and autophagy
Abstract
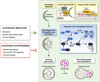
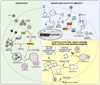
Autophagy is an evolutionarily conserved intracellular process by which bulk cytoplasm is enveloped inside a double-membraned vesicle and shuttled to lysosomes for degradation. Within the last 15 years, the genes necessary for the execution of autophagy have been identified and the number of tools for studying this process has grown. Autophagy is essential for tissue homeostasis and development and defective autophagy is associated with a number of diseases. As intracellular parasites, during the course of an infection, viruses encounter autophagy and interact with the proteins that execute this process. Autophagy and/or autophagy genes likely play both anti-viral and pro-viral roles in the life cycles and pathogenesis of many different virus families. With respect to anti-viral roles, the autophagy proteins function in targeting viral components or virions for lysosomal degradation in a process termed xenophagy, and they also play a role in the initiation of innate and adaptive immune system responses to viral infections. Consistent with this anti-viral role of host autophagy, some viruses encode virulence factors that interact with the host autophagy machinery and block the execution of autophagy. In contrast, other viruses appear to utilise components of the autophagic machinery to foster their own intracellular growth or non-lytic cellular egress. As the details of the role (s) of autophagy in viral pathogenesis become clearer, new anti-viral therapies could be developed to inhibit the beneficial and enhance the destructive aspects of autophagy on the viral life cycle.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

