Reproducibility of serologic assays for influenza virus A (H5N1)
- PMID: 19751587
- PMCID: PMC2815968
- DOI: 10.3201/eid1508.081754
Reproducibility of serologic assays for influenza virus A (H5N1)
Abstract
Hemagglutination-inhibition (HI) and neutralization are used to evaluate vaccines against influenza virus A (H5N1); however, poor standardization leads to interlaboratory variation of results. A candidate antibody standard (07/150) was prepared from pooled plasma of persons given clade 1 A/Vietnam/1194/2004 vaccine. To test human and sheep antiserum, 15 laboratories used HI and neutralization and reassortant A/Vietnam/1194/2004, A/turkey/Turkey/1/2005 (clade 2.2), and A/Anhui/1/2005 (clade 2.3.4) viruses. Interlaboratory variation was observed for both assays, but when titers were expressed relative to 07/150, overall percentage geometric coefficient of variation for A/Vietnam/1194/2004 was reduced from 125% to 61% for HI and from 183% to 81% for neutralization. Lack of reduced variability to clade 2 antigens suggested the need for clade-specific standards. Sheep antiserum as a standard did not reliably reduce variability. The World Health Organization has established 07/150 as an international standard for antibody to clade 1 subtype H5 and has an assigned potency of 1,000 IU/ampoule.
Figures
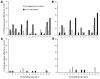
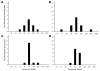
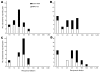
References
-
- Zambon M. Laboratory diagnosis of influenza. In: Nicholson KG, Webster RG, Hay AJ, editors. Influenza. Oxford (UK): Blackwell Publishing; 1997. p. 123–56.
-
- Potter CW. Determinants of immunity to influenza infection in man. Br Med Bull. 1979;35:69–75. - PubMed
-
- Wood JM, Newman RK, Ploss K. The use of correlates of immunity in European Union licensing of influenza vaccines. Dev Biol (Basel). 2003;115:9–16. - PubMed
-
- Committee for Proprietary Medicinal Products. Note for guidance on harmonisation of requirements for influenza vaccines, March 12, 1997; CPMP/BWP/214/96 [cited 2009 May 5]. Available from http://www.emea.europa.eu/pdfs/human/bwp/021496en.pdf
-
- US Food and Drug Administration. Guidance for industry. Clinical data needed to support the licensure of pandemic influenza vaccines [cited 2009 May 5]. Available from http://www.fda.gov/cber/gdlns/panfluvac.pdf
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
