Selective filtering of particles by the extracellular matrix: an electrostatic bandpass
- PMID: 19751661
- PMCID: PMC2749787
- DOI: 10.1016/j.bpj.2009.07.009
Selective filtering of particles by the extracellular matrix: an electrostatic bandpass
Abstract
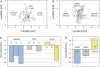
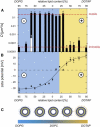
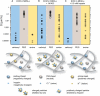
The transport of microscopic particles such as growth factors, proteins, or drugs through the extracellular matrix (ECM) is based on diffusion, a ubiquitous mechanism in nature. The ECM shapes the local distribution of the transported macromolecules and at the same time constitutes an important barrier toward infectious agents. To fulfill these competing tasks, the hydrogels have to employ highly selective filtering mechanisms. Yet, the underlying microscopic principles are still an enigma in cell biology and drug delivery. Here, we show that the extracellular matrix presents an effective electrostatic bandpass, suppressing the diffusive motion of both positively and negatively charged objects. This mechanism allows uncharged particles to easily diffuse through the matrix, while charged particles are effectively trapped. However, by tuning the strength of this physical interaction of the particles with the biopolymer matrix, the microscopic mobility of formerly trapped particles can be rescued on demand. Moreover, we identify heparan sulfate chains to be one important key factor for the barrier function of the extracellular matrix. We propose that localized charge patches in the ECM are responsible for its highly unspecific but strongly selective filtering effect. Such localized interactions could also account for the observed tunability and selectivity of many other important permeability barriers that are established by biopolymer-based hydrogels, e.g., the mucus layer of endothelial cells or the hydrogel in the nuclear core complex.
Figures

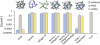

References
-
- Zamecnik J., Vargova L., Homola A., Kodet R., Sykova E. Extracellular matrix glycoproteins and diffusion barriers in human astrocytic tumors. Neuropathol. Appl. Neurobiol. 2003;30:338–350. - PubMed
-
- Kalluri R. Basement membranes: structure, assembly and role in tumor angiogenesis. Nat. Rev. Cancer. 2003;3:422–433. - PubMed
-
- Schittny J.C., Yurchenco P.D. Basement membranes: molecular organization and function in development and disease. Curr. Opin. Cell Biol. 1989;1:983–988. - PubMed
-
- Rosso F., Giordano A., Barbarisi M., Barbarisi A. From cell-ECM interactions to tissue engineering. J. Cell. Physiol. 2004;199:174–180. - PubMed
-
- Taipale J., Keski-Oja J. Growth factors in the extracellular matrix. FASEB J. 1997;11:51–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

