A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5
- PMID: 19752419
- PMCID: PMC2872173
- DOI: 10.1001/archophthalmol.2009.234
A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5
Erratum in
- Arch Ophthalmol. 2009 Dec;127(12):1648
Abstract
Objective: To compare the efficacy and safety of 1-mg and 4-mg doses of preservative-free intravitreal triamcinolone with observation for eyes with vision loss associated with macular edema secondary to perfused central retinal vein occlusion (CRVO).
Methods: Multicenter, randomized, clinical trial of 271 participants.
Main outcome measure: Gain in visual acuity letter score of 15 or more from baseline to month 12.
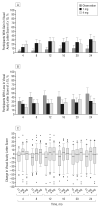
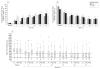
Results: Seven percent, 27%, and 26% of participants achieved the primary outcome in the observation, 1-mg, and 4-mg groups, respectively. The odds of achieving the primary outcome were 5.0 times greater in the 1-mg group than the observation group (odds ratio [OR], 5.0; 95% confidence interval [CI], 1.8-14.1; P = .001) and 5.0 times greater in 4-mg group than the observation group (OR, 5.0; 95% CI, 1.8-14.4; P = .001); there was no difference identified between the 1-mg and 4-mg groups (OR, 1.0; 95% CI, 0.5-2.1; P = .97). The rates of elevated intraocular pressure and cataract were similar for the observation and 1-mg groups, but higher in the 4-mg group.
Conclusions: Intravitreal triamcinolone is superior to observation for treating vision loss associated with macular edema secondary to CRVO in patients who have characteristics similar to those in the SCORE-CRVO trial. The 1-mg dose has a safety profile superior to that of the 4-mg dose. Application to Clinical Practice Intravitreal triamcinolone in a 1-mg dose, following the retreatment criteria applied in the SCORE Study, should be considered for up to 1 year, and possibly 2 years, for patients with characteristics similar to those in the SCORE-CRVO trial. Trial Registration clinicaltrials.gov Identifier: NCT00105027.
Figures
Comment in
-
SCOREing in retinal venous occlusive disease.Arch Ophthalmol. 2009 Sep;127(9):1203-4. doi: 10.1001/archophthalmol.2009.226. Arch Ophthalmol. 2009. PMID: 19752431 No abstract available.
-
Laser photocoagulation and intravitreal injection of triamcinolone for retinal vein occlusions.JAMA. 2009 Oct 21;302(15):1693-5. doi: 10.1001/jama.2009.1523. JAMA. 2009. PMID: 19856489 No abstract available.
References
-
- David R, Zangwill L, Badarna M, Yassur Y. Epidemiology of retinal vein occlusion and its association with glaucoma and increased intraocular pressure. Ophthalmologica. 1988;197(2):69–74. - PubMed
-
- Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. Arch Ophthalmol. 1996;114(10):1243–1247. - PubMed
-
- Klein R, Moss SE, Meuer SM, Klein BEK. The 15-year cumulative incidence of retinal vein occlusion. Arch Ophthalmol. 2008;126(4):513–518. - PubMed
-
- Klein R, Klein BE, Lee KE, Cruickshanks KJ, Gangnon RE. Changes in visual acuity in a population over a 15 year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;142(4):539–549. - PubMed
-
- Central Vein Occlusion Study Group Baseline and early natural history report. Arch Ophthalmol. 1993;111(8):1087–1095. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

