Loss of mitogen-activated protein kinase kinase kinase 4 (MAP3K4) reveals a requirement for MAPK signalling in mouse sex determination
- PMID: 19753101
- PMCID: PMC2733150
- DOI: 10.1371/journal.pbio.1000196
Loss of mitogen-activated protein kinase kinase kinase 4 (MAP3K4) reveals a requirement for MAPK signalling in mouse sex determination
Abstract
Sex determination in mammals is controlled by the presence or absence of the Y-linked gene SRY. In the developing male (XY) gonad, sex-determining region of the Y (SRY) protein acts to up-regulate expression of the related gene, SOX9, a transcriptional regulator that in turn initiates a downstream pathway of testis development, whilst also suppressing ovary development. Despite the requirement for a number of transcription factors and secreted signalling molecules in sex determination, intracellular signalling components functioning in this process have not been defined. Here we report a role for the phylogenetically ancient mitogen-activated protein kinase (MAPK) signalling pathway in mouse sex determination. Using a forward genetic screen, we identified the recessive boygirl (byg) mutation. On the C57BL/6J background, embryos homozygous for byg exhibit consistent XY gonadal sex reversal. The byg mutation is an A to T transversion causing a premature stop codon in the gene encoding MAP3K4 (also known as MEKK4), a mitogen-activated protein kinase kinase kinase. Analysis of XY byg/byg gonads at 11.5 d post coitum reveals a growth deficit and a failure to support mesonephric cell migration, both early cellular processes normally associated with testis development. Expression analysis of mutant XY gonads at the same stage also reveals a dramatic reduction in Sox9 and, crucially, Sry at the transcript and protein levels. Moreover, we describe experiments showing the presence of activated MKK4, a direct target of MAP3K4, and activated p38 in the coelomic region of the XY gonad at 11.5 d post coitum, establishing a link between MAPK signalling in proliferating gonadal somatic cells and regulation of Sry expression. Finally, we provide evidence that haploinsufficiency for Map3k4 accounts for T-associated sex reversal (Tas). These data demonstrate that MAP3K4-dependent signalling events are required for normal expression of Sry during testis development, and create a novel entry point into the molecular and cellular mechanisms underlying sex determination in mice and disorders of sexual development in humans.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
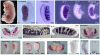
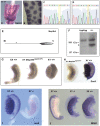

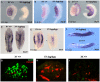

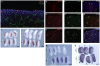

References
-
- Brennan J, Capel B. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet. 2004;5:509–521. - PubMed
-
- Gubbay J, Collignon J, Koopman P, Capel B, Economou A, et al. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–250. - PubMed
-
- Koopman P, Münsterberg A, Capel B, Vivian N, Lovell-Badge R. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature. 1990;348:450–452. - PubMed
-
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. - PubMed
-
- Lovell-Badge R, Robertson E. XY female mice resulting from a heritable mutation in the murine primary testis determining gene, Tdy. Development. 1990;109:635–646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MC_UP_1502/1/MRC_/Medical Research Council/United Kingdom
- MC_U142684175/MRC_/Medical Research Council/United Kingdom
- MC_U142670371/MRC_/Medical Research Council/United Kingdom
- U.1426.00.004.00001.01/MRC_/Medical Research Council/United Kingdom
- MC_U142684172/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

