The functional network of ion channels in T lymphocytes
- PMID: 19754890
- PMCID: PMC3133616
- DOI: 10.1111/j.1600-065X.2009.00816.x
The functional network of ion channels in T lymphocytes
Abstract
For more than 25 years, it has been widely appreciated that Ca2+ influx is essential to trigger T-lymphocyte activation. Patch clamp analysis, molecular identification, and functional studies using blockers and genetic manipulation have shown that a unique contingent of ion channels orchestrates the initiation, intensity, and duration of the Ca2+ signal. Five distinct types of ion channels--Kv1.3, KCa3.1, Orai1+ stromal interacting molecule 1 (STIM1) [Ca2+-release activating Ca2+ (CRAC) channel], TRPM7, and Cl(swell)--comprise a network that performs functions vital for ongoing cellular homeostasis and for T-cell activation, offering potential targets for immunomodulation. Most recently, the roles of STIM1 and Orai1 have been revealed in triggering and forming the CRAC channel following T-cell receptor engagement. Kv1.3, KCa3.1, STIM1, and Orai1 have been found to cluster at the immunological synapse following contact with an antigen-presenting cell; we discuss how channels at the synapse might function to modulate local signaling. Immuno-imaging approaches are beginning to shed light on ion channel function in vivo. Importantly, the expression pattern of Ca2+ and K+ channels and hence the functional network can adapt depending upon the state of differentiation and activation, and this allows for different stages of an immune response to be targeted specifically.
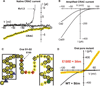
Figures






References
-
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. - PubMed
-
- DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984;307:465–468. - PubMed
-
- Matteson DR, Deutsch C. K channels in T lymphocytes: a patch clamp study using monoclonal antibody adhesion. Nature. 1984;307:468–471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

