Characterisation of a live Salmonella vaccine stably expressing the Mycobacterium tuberculosis Ag85B-ESAT6 fusion protein
- PMID: 19755145
- PMCID: PMC2789253
- DOI: 10.1016/j.vaccine.2009.09.007
Characterisation of a live Salmonella vaccine stably expressing the Mycobacterium tuberculosis Ag85B-ESAT6 fusion protein
Abstract
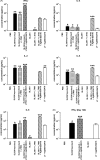
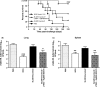
A recombinant Salmonella enterica serovar Typhimurium (S. Typhimurium) vaccine strain was constructed that stably expressed the Mycobacterium tuberculosis fusion antigen Ag85B-ESAT6 from the chromosome. Live oral vaccination of mice with the Salmonella/Ag85B-ESAT6 strain generated a potent anti-Ag85B-ESAT6 T(H)1 response with high antibody titres with a IgG2a-bias and significant IFN-gamma production lasting over a 120-day period. When mice primed with the Salmonella/Ag85B-ESAT6 vaccine were mucosally boosted with the Ag85B-ESAT6 antigen and adjuvant the IFN-gamma responses increased markedly. To determine the protective efficacy of this vaccine strain, guinea pigs were immunised and followed for a 30-week period after aerosol challenge with M. tuberculosis. The heterologous prime-boost strategy of live Salmonella vaccine followed by a systemic boost of antigen and adjuvant reduced the levels of M. tuberculosis bacteria in the lungs and spleen to the same extent as BCG. Additionally, this vaccination regimen was observed to be statistically equivalent in terms of protection to immunisation with BCG. Thus, live oral priming with the recombinant Salmonella/Ag85B-ESAT6 and boosting with Ag85B-ESAT6 plus the adjuvant LTK63 represents an effective mucosal vaccination regimen.
Figures


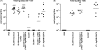

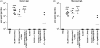
Similar articles
-
Fusion protein Ag85B-MPT64(190-198)-Mtb8.4 has higher immunogenicity than Ag85B with capacity to boost BCG-primed immunity against Mycobacterium tuberculosis in mice.Vaccine. 2009 Oct 19;27(44):6179-85. doi: 10.1016/j.vaccine.2009.08.018. Epub 2009 Aug 25. Vaccine. 2009. PMID: 19712772
-
Immunogenicity and protective efficacy of a fusion protein vaccine consisting of antigen Ag85B and HspX against Mycobacterium tuberculosis infection in mice.Scand J Immunol. 2011 Jun;73(6):568-76. doi: 10.1111/j.1365-3083.2011.02531.x. Scand J Immunol. 2011. PMID: 21323695
-
Mucosal administration of Ag85B-ESAT-6 protects against infection with Mycobacterium tuberculosis and boosts prior bacillus Calmette-Guerin immunity.J Immunol. 2006 Nov 1;177(9):6353-60. doi: 10.4049/jimmunol.177.9.6353. J Immunol. 2006. PMID: 17056566
-
Novel tuberculosis vaccines based on TB10.4 and Ag85B: State-of-art and advocacy for good practices.Vaccine. 2025 Apr 19;53:126932. doi: 10.1016/j.vaccine.2025.126932. Epub 2025 Mar 2. Vaccine. 2025. PMID: 40031085 Review.
-
DNA and RNA vaccines against tuberculosis: a scoping review of human and animal studies.Front Immunol. 2024 Oct 3;15:1457327. doi: 10.3389/fimmu.2024.1457327. eCollection 2024. Front Immunol. 2024. PMID: 39421744 Free PMC article.
Cited by
-
Mucosal delivery of tuberculosis vaccines: a review of current approaches and challenges.Expert Rev Vaccines. 2019 Dec;18(12):1271-1284. doi: 10.1080/14760584.2019.1692657. Epub 2019 Dec 26. Expert Rev Vaccines. 2019. PMID: 31876199 Free PMC article. Review.
-
Antigen Localization Influences the Magnitude and Kinetics of Endogenous Adaptive Immune Response to Recombinant Salmonella Vaccines.Infect Immun. 2017 Nov 17;85(12):e00593-17. doi: 10.1128/IAI.00593-17. Print 2017 Dec. Infect Immun. 2017. PMID: 28893919 Free PMC article.
-
Immunotherapeutic efficacy of recombinant Mycobacterium smegmatis expressing Ag85B-ESAT6 fusion protein against persistent tuberculosis infection in mice.Hum Vaccin Immunother. 2014;10(1):150-8. doi: 10.4161/hv.26171. Epub 2013 Aug 27. Hum Vaccin Immunother. 2014. PMID: 23982126 Free PMC article.
-
Antitumor effect of oral cancer vaccine with Bifidobacterium delivering WT1 protein to gut immune system is superior to WT1 peptide vaccine.Hum Vaccin Immunother. 2018 Jan 2;14(1):159-162. doi: 10.1080/21645515.2017.1382787. Epub 2017 Oct 30. Hum Vaccin Immunother. 2018. PMID: 29048978 Free PMC article.
-
Live-Attenuated Bacterial Vectors: Tools for Vaccine and Therapeutic Agent Delivery.Vaccines (Basel). 2015 Nov 10;3(4):940-72. doi: 10.3390/vaccines3040940. Vaccines (Basel). 2015. PMID: 26569321 Free PMC article. Review.
References
-
- WHO. Joint HIV/tuberculosis interventions. 2007.
-
- WHO. Tuberculosis. Fact Sheet No 104. 2007. [cited; Available from: http://www.who.int/mediacentre/factsheets/fs104/en/].
-
- Colditz G.A., Brewer T.F., Berkey C.S., Wilson M.E., Burdick E., Fineberg H.V. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271(March 2 (9)):698–702. - PubMed
-
- Dietrich J., Andersen C., Rappuoli R., Doherty T.M., Jensen C.G., Andersen P. Mucosal administration of Ag85B–ESAT-6 protects against infection with Mycobacterium tuberculosis and boosts prior bacillus Calmette-Guerin immunity. J Immunol. 2006;177(November 1 (9)):6353–6360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

